Continuous Bioprocessing Market Overview and Key Insights:
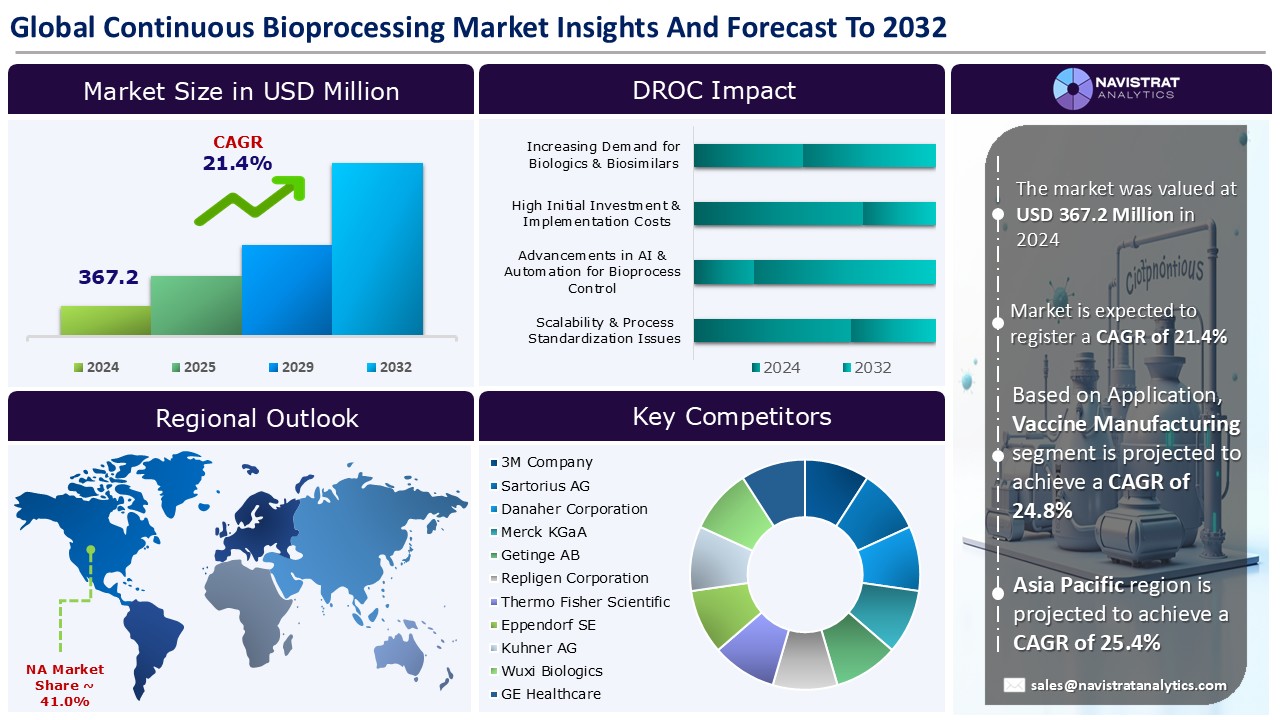
The continuous bioprocessing market size reached USD 367.2 million in 2024 and is expected to register a revenue CAGR of 21.4% during the forecast period. Continuous bioprocessing is a single-unit operation in biomanufacturing in which raw materials are constantly loaded, processed, and unloaded.
Market Drivers:
Increasing demand for biologics & biosimilars is a key driver of revenue growth in the continuous bioprocessing market. Continuous Manufacturing (CM) has the advantage of streamlined processes and a smaller manufacturing facility footprint for producing large volumes of low-cost, high-quality biopharmaceuticals. Another possible benefit of continuous bioprocessing and manufacturing is that it can improve end-product quality and consistency, owing to the need for more strict controls and monitoring. The use of process analytical technologies (PAT) gives operators more control over the cellular environment and metabolism, as well as the overall production environment, which should improve control over product quality.
Furthermore, rising adoption of single-use technologies is also driving the market revenue growth. Single-use systems represent the future of biopharmaceutical drug processing, offering substantial advantages over traditional reusable stainless-steel systems and partially disposable systems. Single-use systems enhance sustainability by eliminating the chemicals and resources, such as water and electricity, required to sterilize reusable systems.
On June 2024, WuXi Biologics, a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), announced that three sets of 5,000L single-use bioreactors have been successfully installed in the second drug substance line at its Hangzhou manufacturing facility, which will begin operations in 2021. With the GMP production of the newly installed bioreactors, the facility’s overall capacity will be expanded from 8,000L to 23,000L, further strengthening the company’s manufacturing capability for global clients.
Market Opportunity:
Advancements in AI & automation for bioprocess control acts as opportunities for continuous bioprocessing market. Bioprocessing professionals have been using AI techniques such as pattern recognition, machine learning algorithms (e.g., multivariate data analysis or more complex deep neural networks), text mining, natural language processing (NLP), and other signal processing methods for real-time process monitoring to understand critical process parameters such as mixing efficiency and cell growth rates at various stages of cell culture.
Digital twins are preferred over experimental procedures in bioprocessing because they provide more and superior data, as well as sound and scientific decision-making throughout the product lifecycle. As a result, they can be used as an early detection technique for changes in the states of both systems, regardless of any precise variations in real values.
Recent Trends:
Emerging trends include integration of AI and automation, adoption of single-use technologies, and expansion beyond monoclonal antibodies.
The integrated continuous manufacturing process not only increases productivity and reduces contamination, but it also establishes a generic platform for continuous production of viral vectored vaccines. Adherent Vero cells are frequently employed as a continuous cell line in the production of several viral vaccines, including those for Ebola, influenza, and rabies. The Vero cell line has been shown to be a very vulnerable cell line to viral infection.
On March 2022, Spectrum Chemical Mfg. Corp., a global leader in fine chemicals, laboratory equipment, and supplies, announced the addition of 12 new products to its rapidly expanding bioCERTIFIED quality management system and chemicals range for biopharmaceutical manufacture. The 12 new bioCERTIFIED products comprise sugars/carbohydrates, organic and inorganic salts, and preservatives that are employed in a variety of bioprocessing applications, from upstream cell culture development to downstream purification, polishing, and final fill manufacturing.
Restraints & Challenges:
High initial investment & implementation costs is a key restraining factor for growth of continuous bioprocessing market. The usage of PAT and automation software increases the cost of developing continuous processes. The high initial cost is another critical hurdle for enterprises in implementing continuous manufacturing, because it is difficult to justify the need for new equipment while existing batch equipment is still functional with proven regulatory approval.
Additionally, CM technology development and implementation in cGMP facilities require highly skilled personnel. Although training has been conducted, addressing the knowledge gap would require greater multidisciplinary collaboration and commitment from all relevant stakeholders. This is a challenge for manufacturers and regulators alike, as CM is still in its infancy in the pharmaceutical industry, leaving a shortage of personnel with the necessary skills and knowledge. Continuous systems require statistically trained personnel to understand the data generated.
Product Type Segment Insights and Analysis:
Based on the product type, the continuous bioprocessing market is segmented into bioreactors, filtration systems, chromatography systems, process analytical technologies, and consumables & reagents.
Bioreactors segment contributed the largest market share in 2024. The core of pharmaceutical bioprocessing, bioreactors are essential to the creation of biopharmaceuticals. The future of bioreactor technology is being shaped by advancements in continuous bioprocessing, sophisticated sensors, smart bioreactors, modular designs, and sustainable practices, even though operational challenges including scale-up issues, process monitoring, and regulatory compliance still exist.
Bioreactors will become more effective, adaptable, and sustainable as these developments progress, improving the biopharmaceutical industry’s capacity to satisfy the rising need for novel treatments. Perfusion bioreactors have been used for decades to run mammalian cell culture procedures continuously. Using extended control systems based on the metabolic needs of the cell line for continuous perfusion systems could result in notable productivity gains.
On August 2023, Sartorius and Repligen Corporation have announced the introduction of an integrated bioreactor system that incorporates Repligen XCell ATF upstream intensification technology into Sartorius’ Biostat STR bioreactor, simplifying intensified seed train and N perfusion implementation for biopharmaceutical manufacturers. The Biostat STR now has a completely compatible embedded XCell ATF hardware and software module that provides established advanced control recipes with integrated Process Analytical Technology (PAT).
Process Type Segment Insights and Analysis:
Based on the process type, the continuous bioprocessing market is segmented into upstream processing, downstream processing, and end-to-end continuous bioprocessing.
Downstream processing segment contributed the largest market share in 2024. Although continuous production has been used in a variety of industries, biotechnology, where batchwise processing is still the norm, has only grudgingly adopted it. Development is utilizing a combination of upstream perfusion, continuous downstream processes using Continuous Multi-Column Chromatography (MCC), in-line conditioning, and tangential flow filtration (TFF) for products that are challenging to produce using conventional technology—often because of stability problems or cytotoxic/cytostatic effects on cell culture.
On May 2024, Sartorius has announced a collaboration with the biopharmaceutical giant Sanofi to create an end-to-end platform for integrated and continuous downstream bioprocessing. Sartorius has been selected as a preferred supplier and will offer its engineering and manufacturing skills to commercialize ICB platforms based on Sanofi prototypes. In exchange, Sanofi will provide Sartorius with exclusive access to its ICB platform know-how and patents. Sartorius will solely commercialize the ICB platform offering to clients worldwide under the terms of the collaboration and license agreement.
Application Segment Insights and Analysis:
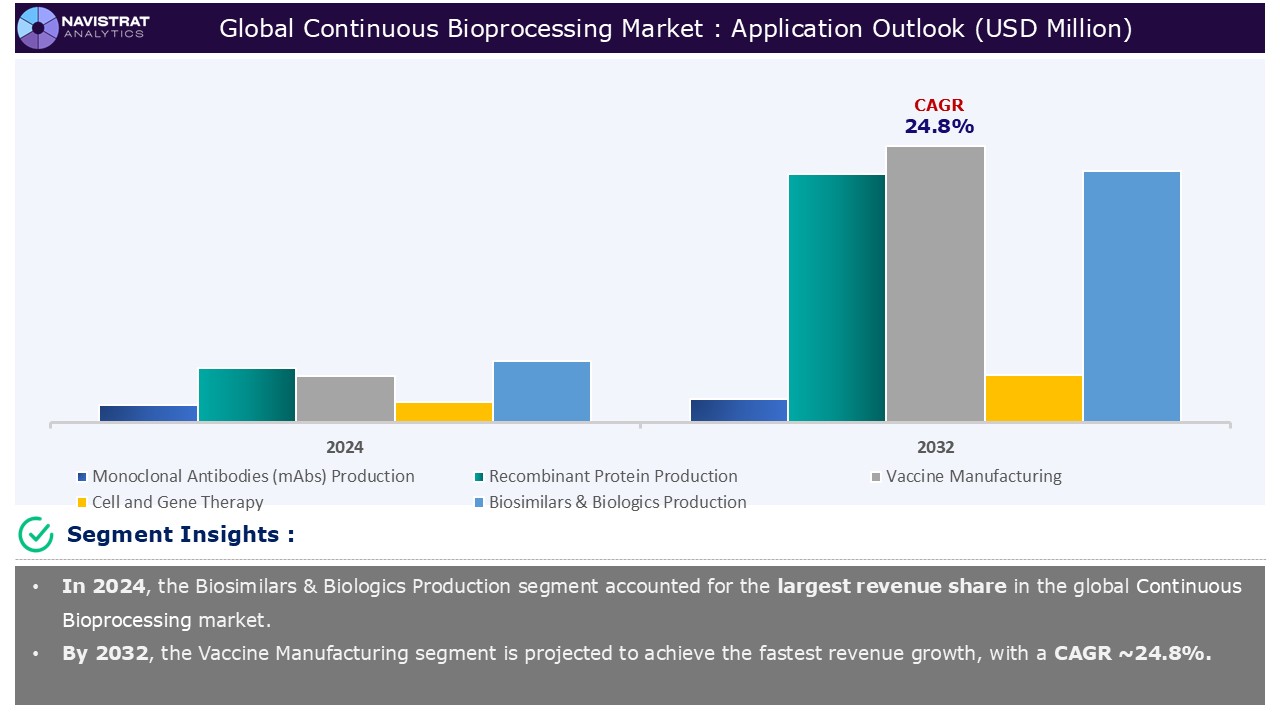
Based on the application, the continuous bioprocessing market is segmented into Monoclonal Antibodies (mAbs) production, recombinant protein production, vaccine manufacturing, cell and gene therapy, and biosimilars & biologics production.
Recombinant protein production segment contributed the largest market share in 2024. Continuous biomanufacturing of recombinant therapeutic proteins has various potential benefits over traditional batch processing, including lower costs, more flexible and responsive manufacturing facilities, and enhanced and consistent product quality.
These include the use of high-throughput devices for effective bioprocess optimization as well as disposable systems, continuous upstream processing, continuous chromatography, integrated continuous bioprocessing, Quality by design, and process analytical technologies to achieve quality products with higher yields. Researchers have achieved significantly higher levels of process intensification and have begun to integrate various continuous unit operations into bigger, holistic processes.
On November 2023, WuXi Biologics, a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), announced that it has completed end-to-end DS manufacturing on a pilot scale using WuXiUP, a proprietary ultra-high productivity continuous bioprocessing platform, at its non-GMP pilot plant in Shanghai. This well-configured bioprocess is now being scaled up to GMP manufacturing and will be used across WuXi Biologics’ global manufacturing sites in China, Ireland, the United States, and Singapore.
Vaccine is expected to register the fastest growth rate during the forecasted period. Biomanufacturers have made substantial investments in continuous manufacturing development because it has the potential to minimize process steps, footprint, and equipment size while improving product quality and lowering costs.
Although the use of continuous processing is still in its early phases, the vaccine business has embraced it and is eager to investigate the full benefits of this method. Biomanufacturers (including vaccine developers) are increasingly adopting quality by design (QbD) principles. QbD stresses process comprehension and control, which are also necessary for continuous processing, particularly the deployment of process control techniques as appropriate analytical tools for successful continuous production.
On July 2023, a three-year research study led by MIT scientists announced that it intends to build the world’s first fully integrated, continuous mRNA manufacturing platform, with a USD 82 million grant from the US Food and Drug Administration’s Center for Biologics Evaluation and Research. The resulting pilot-scale system is intended to improve society’s ability to respond to future pandemics while also accelerating the development and production of mRNA technologies, which companies are investing in at unprecedented scales in the hopes of developing new vaccines and treatments for cancers, metabolic disorders, genetic diseases, and other conditions.
Geographical Outlook:
Continuous bioprocessing market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Continuous Bioprocessing Market:
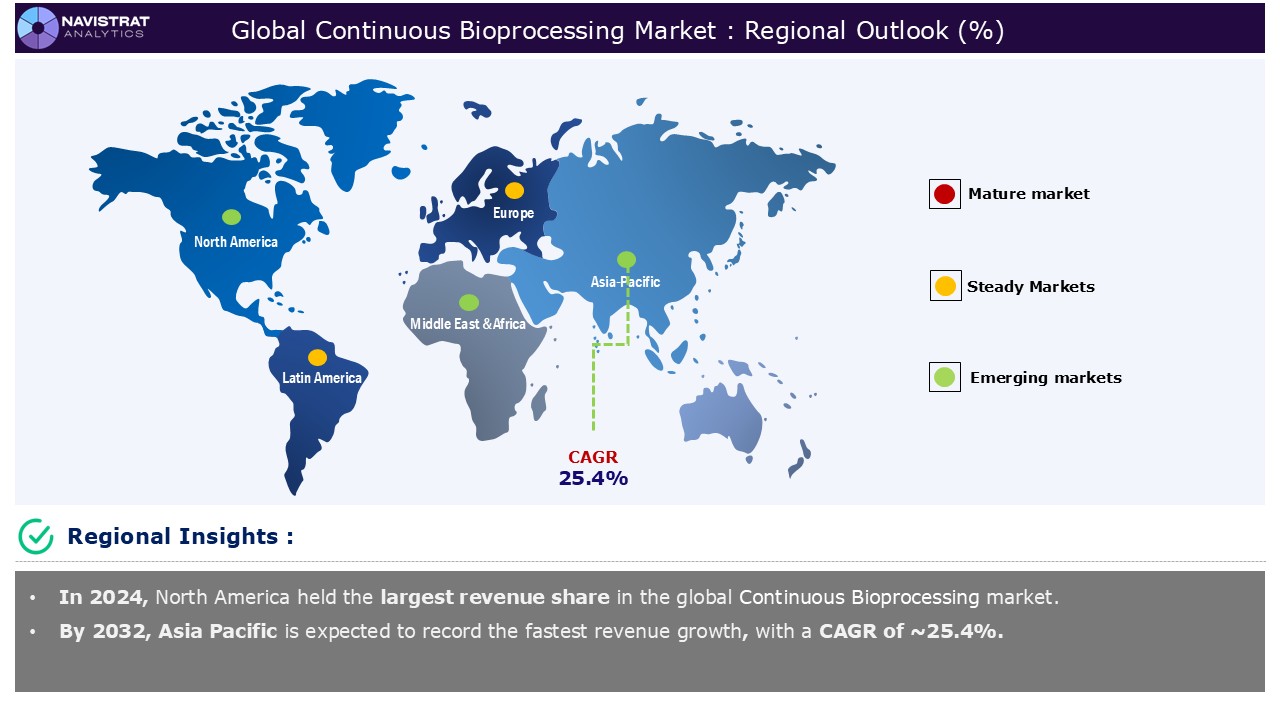
North America is registered to have highest market share in continuous bioprocessing market in 2024. This is mainly due to the increasing demand for biologics & biosimilars and rising adoption of single-use technologies. Patients can benefit from both biosimilars and continuous manufacturing since they reduce the cost of key biopharmaceuticals. The decreased risk profile of biosimilars makes the risks associated with developing and launching new biosimilar products based on CM technology more manageable and acceptable.
On March 2025, Pow.Bio, a pioneer in creative biomanufacturing solutions, announced the inauguration of a new cutting-edge facility in Alameda, CA. The new 25,000-square-foot facility, located in Marina Village in Alameda, features cutting-edge production facilities outfitted with AI-enabled continuous fermentation capabilities, allowing partners to meet target production costs for competitive manufacturing. The facility houses a fleet of dual-chamber continuous systems of varying sizes, demonstrating Pow.Bio’s unique approach to AI-enabled biomanufacturing.
Asia Pacific Continuous Bioprocessing Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period, driven by rising adoption of personalized medicine & cell & gene therapy and increasing outsourcing to CDMOs & CMOs. Several Asian-Pacific local governments have established beneficial policies and guidelines in the last five years to enhance healthcare and accelerate drug research in their respective markets. The Healthy China 2030 project is one example of a strategy for meeting the demands of an aging population. It comprises efforts like as reducing the approval process for new pharmaceutical goods, hastening their market debut, and incorporating these treatments into medical insurance systems.
On October 2024, Thermo Fisher Scientific and the Government of Telangana have signed a Memorandum of Understanding (MoU) to build a Bioprocess Design Centre (BDC) in Genome Valley, Hyderabad. The 10,000-square-foot Bioprocess Design Centre is expected to open in early 2025. This facility will improve biotherapeutic development and production in India and the Asia-Pacific region by providing laboratories and training hubs to fuel scientific research and innovation.
Europe Continuous Bioprocessing Market:
Europe is expected to have considerable market share in 2024. This is mainly due to rising adoption of personalized medicine & cell & gene therapy, increasing demand for biologics & biosimilars, and technological advancements in bioprocessing. Pharmaceuticals, including biopharmaceuticals, are Europe’s second largest industrial sector in terms of exports. Biopharmaceutical manufacturing is a high-value business in Europe. Biopharmaceuticals now account for more than half of the pharmaceutical market in both Europe and the United States. The lower cost of continuous biomanufacturing results in a lower environmental footprint, which, in view of the EU Green Deal goals for 2030 and 2050, and the associated need to significantly reduce carbon footprint/environmental footprint, is a clear motivator for change.
Competition Analysis:
The continuous bioprocessing market is characterized by a fragmented structure, with several players competing across various segments and regions. list of major players included in the continuous bioprocessing market report are:
- 3M Company
- Sartorius AG
- Danaher Corporation
- Merck KGaA
- Getinge AB
- Repligen Corporation
- Thermo Fisher Scientific
- Eppendorf SE
- Kuhner AG
- Wuxi Biologics
- GE Healthcare
- Asahi Kasei Bioprocess America, Inc.
- Evotec Biologics
- Esco Group of Companies
- FUJIFILM Holdings Corporation
Strategic Developments in Continuous Bioprocessing Market:
- In February 2025, uFraction8 Limited, a leading microfluidics-based bio-separation technology provider, has closed a GBP 3.4 million funding round led by the Ventures team at Foresight Group, with participation from Old College Capital, Scottish Enterprise, Alwyn Capital, and existing investor Thia Ventures. The round includes a GBP 1.5 million grant from the Polish Agency for Enterprise Development (PARP).
- In December 2024, 908 Devices Inc. and Getinge have announced a collaboration to connect Getinge’s bioreactors with 908 Devices’ MAVEN for automatic glucose and lactate control in cell cultures. The combined approach will allow scientists to measure important process parameters in bioreactors in real time, eliminating the need for manual sampling. MAVEN from 908 Devices delivers very precise, on-line monitoring and control of glucose and lactate without reducing bioreactor volume, allowing biopharma experts to maintain appropriate nutrition and metabolite concentrations even at low levels.
- In June 2024, Waters Corporation and Sartorius have announced a new collaboration to develop integrated analytical solutions for downstream biomanufacturing, expanding their previous arrangement that focused on upstream bioprocessing analytics. Software and hardware integrations between the Waters PATROL UltraPerformance Liquid Chromatography (UPLC) Process Analysis System and the Sartorius Resolute BioSMB multi-column chromatography platform will provide bioprocess engineers with more comprehensive analytical data for downstream batch and continuous manufacturing, increasing yields while reducing waste and lowering biomanufacturing costs.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Continuous Bioprocessing Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 367.2 Million |
| Market Growth Rate in CAGR (2025–2032) | 21.4% |
| Market Revenue forecast to 2032 | USD 1,740.0 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Explore options (Forms) |
The Continuous Bioprocessing market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Product Type Outlook (Revenue, USD Million; 2022-2032)
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Bioreactors
- Process Type Outlook (Revenue, USD Million; 2022-2032)
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Application Outlook (Revenue, USD Million; 2022-2032)
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- End-Use Outlook (Revenue, USD Million; 2022-2032)
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Continuous Bioprocessing market report
The market size of continuous bioprocessing market was 367.2 million in 2024.
The market size of continuous bioprocessing market is expected to register compound annual growth rate (CAGR) of 21.4% over the forecast period.
Increasing demand for biologics & biosimilars, rising adoption of single-use technologies, and technological advancements in bioprocessing are major key factors driving the market revenue growth of the continuous bioprocessing market.
High initial investment & implementation costs and regulatory challenges & compliance issues are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 25.4%.
Biosimilars & biologics production is the major leading segment of Continuous Bioprocessing market in terms of application.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Applications
- Primary
- Secondary
- Market Size Estimation
- Top-down Application
- Bottom-up Application
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Increasing demand for biologics & biosimilars
- Rising adoption of single-use technologies
- Technological advancements in bioprocessing
- Market Restraints
- High initial investment & implementation costs
- Regulatory challenges & compliance issues
- Market Opportunities
- Advancements in AI & automation for bioprocess control
- Rising adoption of personalized medicine & cell & gene therapy
- Increasing outsourcing to CDMOs & CMOs
- Market Challenges
- Scalability & process standardization issues
- Supply chain disruptions
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Product Type Market Revenue Estimates and Forecasts, 2022-2032
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactor
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Bioreactors
- Process Type Market Revenue Estimates and Forecasts, 2022-2032
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Application Market Revenue Estimates and Forecasts, 2022-2032
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- End-Use Market Revenue Estimates and Forecasts, 2022-2032
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Continuous Bioprocessing Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
-
- North America
- North America Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- North America Continuous Bioprocessing Market By Process Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- North America Continuous Bioprocessing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- North America Continuous Bioprocessing Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- North America Continuous Bioprocessing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- Bioreactors
- North America Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- North America
- Europe
- Europe Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Europe Continuous Bioprocessing Market By Process Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Europe Continuous Bioprocessing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- Europe Continuous Bioprocessing Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Europe Continuous Bioprocessing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Bioreactors
- Europe Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia-Pacific
- Asia-Pacific Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Asia-Pacific Continuous Bioprocessing Market By Process Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Asia-Pacific Continuous Bioprocessing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- Asia-Pacific Continuous Bioprocessing Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Asia-Pacific Continuous Bioprocessing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Bioreactors
- Asia-Pacific Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Latin America Continuous Bioprocessing Market By Process Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Latin America Continuous Bioprocessing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- Latin America Continuous Bioprocessing Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Latin America Continuous Bioprocessing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Bioreactors
- Latin America Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East & Africa
-
- Middle East & Africa Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Bioreactors
- Column Bioreactor
- Tubular Bioreactor
- Stirred-Tank Bioreactor
- Permeable Wall bioreactors
- Perfusion Bioreactor
- Others
- Filtration Systems
- Tangential Flow Filtration (TFF)
- Depth filtration
- Microfiltration
- Ultrafiltration
- Chromatography Systems
- Continuous chromatography
- Simulated Moving Bed (SMB) chromatography
- Process Analytical Technologies
- Consumables & Reagents
- Chemically defined media
- Supplements & growth factors
- Tubing & connectors
- Bags & containers
- Others
- Middle East & Africa Continuous Bioprocessing Market By Process Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Upstream Processing
- Downstream Processing
- End-to-End Continuous Bioprocessing
- Middle East & Africa Continuous Bioprocessing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Monoclonal Antibodies (mAbs) Production
- Recombinant Protein Production
- Vaccine Manufacturing
- Cell and Gene Therapy
- Biosimilars & Biologics Production
- Middle East & Africa Continuous Bioprocessing Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Pharmaceutical & Biotechnology Companies
- CDMO and CRO
- Academic & Research Institutions
- Middle East & Africa Continuous Bioprocessing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Bioreactors
- Middle East & Africa Continuous Bioprocessing Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- 3M Company
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sartorius AG
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Danaher Corporation
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Merck KGaA
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Getinge AB
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Repligen Corporation
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Thermo Fisher Scientific
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Eppendorf SE
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Kuhner AG
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Wuxi Biologics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- GE Healthcare
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Asahi Kasei Bioprocess America, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Evotec Biologics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Esco Group of Companies
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- FUJIFILM Holdings Corporation
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

