Nucleic Acid Therapeutics CDMO Market Overview and Key Insights:
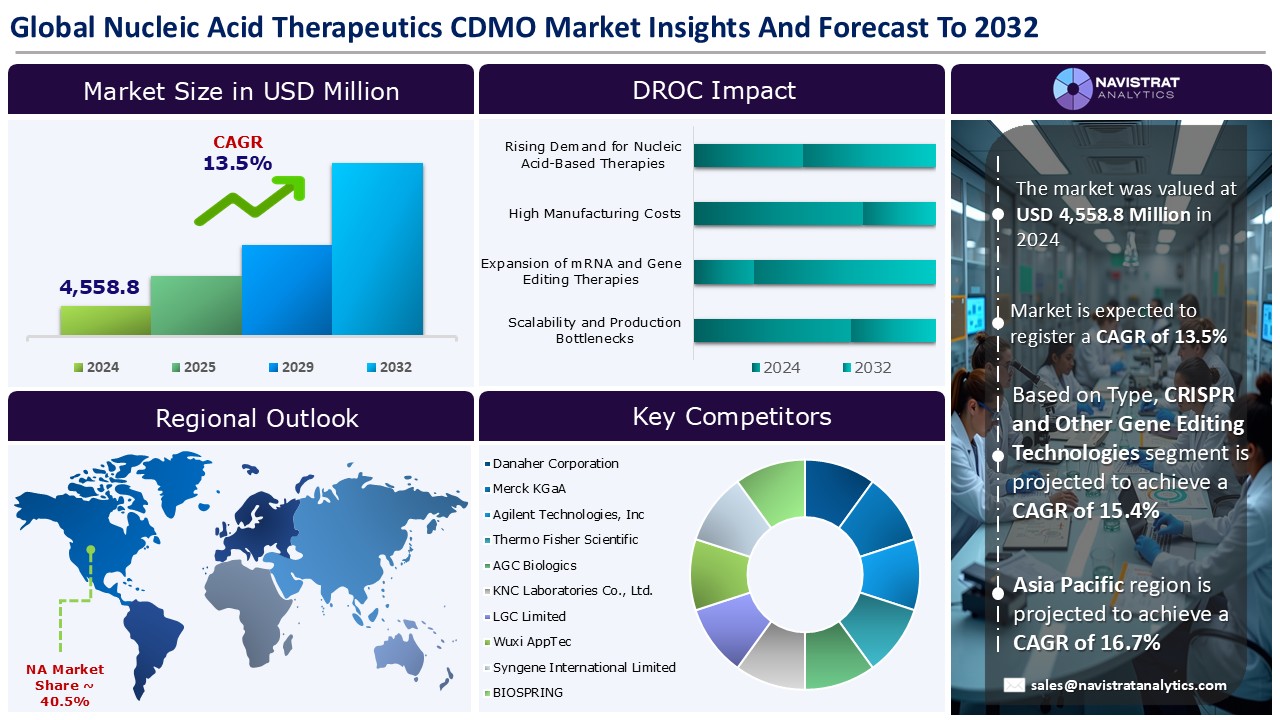
The nucleic acid therapeutics CDMO market size reached USD 4,558.8 million in 2024 and is expected to register a revenue CAGR of 13.5% during the forecast period. The ability of Nucleic Acid Therapeutics (NAT) to target both coding and non-coding sequences has gained more attention in recent years, and regulatory bodies have approved several nucleic acid modalities for therapeutic use, including siRNA, mRNA, aptamer, and antisense oligo.
Market Drivers:
Rising demand for nucleic acid-based therapies is a key driver of revenue growth in the nucleic acid therapeutics CDMO market. Nucleotide-based therapies have enormous potential for selectively modulating cellular pathways in ways that were not before feasible. These therapies comprise all nucleic-acid-based techniques that function inside cells to influence gene expression – the genetic blueprint of disease – with the goal of changing protein expression and potentially altering disease progression.
Nucleic acid medicines have the powerful mix of target selectivity and adaptability needed to enhance therapeutic development for numerous diseases. The tremendous improvement in anti-cancer therapeutic techniques based on NATs has been made possible by advances in drug delivery technologies. Since COVID-19, the field of NATs has grown fast, with a focus on mRNA cancer vaccines. Currently, viral vectors, DNA nanostructures, inorganic nanoparticles, and lipid nanoparticles (LNPs) are frequently used as nucleic acid carriers, with LNPs being the most extensively explored as carriers for mRNA vaccines.
On February 2025, Africa’s EVA Pharma, France’s DNA Script, and Belgium’s Quantoom Biosciences signed a Memorandum of Understanding in Cairo on the sidelines of the African Union Member States’ 2nd Vaccine and Other Health Products Manufacturing Forum, which was organized by Africa CDC and hosted by the Egyptian United Procurement Authority (UPA). This collaboration intends to create the first-ever digital-to-biologics, end-to-end mRNA production platform, with an emphasis on nucleic acid-based vaccines and therapies, such as mRNA and saRNA vaccines for human and animal health.
Market Opportunity:
Expansion of mRNA and gene editing therapies acts as opportunities for nucleic acid therapeutics CDMO market. mRNA appears to be a very promising and innovative treatment method for disorders linked with functional protein loss, by the introduction of a synthetic mRNA that stimulates protein reestablishment and restores function. Nucleic acid-encoded mAbs, particularly mRNA-based monoclonal antibodies, hold significant promise for enhancing antibody treatment efficacy, and targeted cells are used as factories to convert nucleic acids into functional mAbs.
mRNA cancer vaccine platforms have been developed and have shown promising results due to their unique efficacy in advancing the cancer immunity cycle and safety. mRNA vaccines for melanoma, glioblastoma, AML, and renal cell cancer (RCC) showed an active response to immunotherapy, necessitating more research in the mRNA vaccine sector.
On September 2024 Primrose Bio, a leading biotechnology company focused on enhancing the manufacturing of next-generation therapeutics, has partnered with ExPLoRNA Therapeutics, an innovative biotech firm specializing in mRNA technologies, vaccines, and therapeutics. This strategic collaboration aims to co-develop and commercialize products for mRNA medicines. This innovation addresses the stringent manufacturing requirements for a broad range of mRNA-based therapeutics and vaccines.
Recent Trends:
Emerging trends include growth in mRNA and RNA-based therapeutics, increased investment in advanced manufacturing technologies, and increasing demand from biotechnology companies.
Small nucleic acid medications, made from nucleotides, are a unique class of pharmaceuticals that differ dramatically from traditional small molecule and antibody-based therapies. These medicines work by selectively targeting specific genes or messenger RNAs (mRNAs), which modulates gene expression and regulates translational processes. Small interfering RNA (siRNA) treatments can selectively detect and destroy target mRNAs, allowing for precise regulation of gene expression. In recent years, siRNA therapeutics have shown therapeutic potential in cancer, viral infections, neurological disorders, and tumor treatments.
On October 2023, Aldena Therapeutics, a private biotech company pioneering siRNA-based therapies for dermatological indications, has selected PCI Pharma Services (PCI), a world-leading global contract development and manufacturing organization (CDMO), to formulate, package, and distribute ALD-102, a siRNA-based investigational injectable treatment for inflammatory dermatological conditions, all in-house at PCI’s San Diego facility. ALD-102 is now in preclinical trials and is the most advanced medicine in Aldena’s pipeline.
Restraints & Challenges:
The effective distribution of NA molecules to target cells and tissues is one of the major concerns in the field of NA therapies. The efficacy of delivery methods is hampered by the delicate nature of RNA, its susceptibility to nucleases, and the existence of numerous biological barriers. Unintentional immune response activation and interactions between NA therapies and non-targeted cells can have negative consequences and reduce the effectiveness of treatment.
Additionally, unaltered short nucleic acid medications, particularly Antisense Oligonucleotides Therapeutics Market (ASOs), are readily induced by the immune system in addition to being readily broken down by the body’s nucleases. Additionally, negatively charged siRNA medications have a hard time entering cells and working without the aid of a specific delivery method.
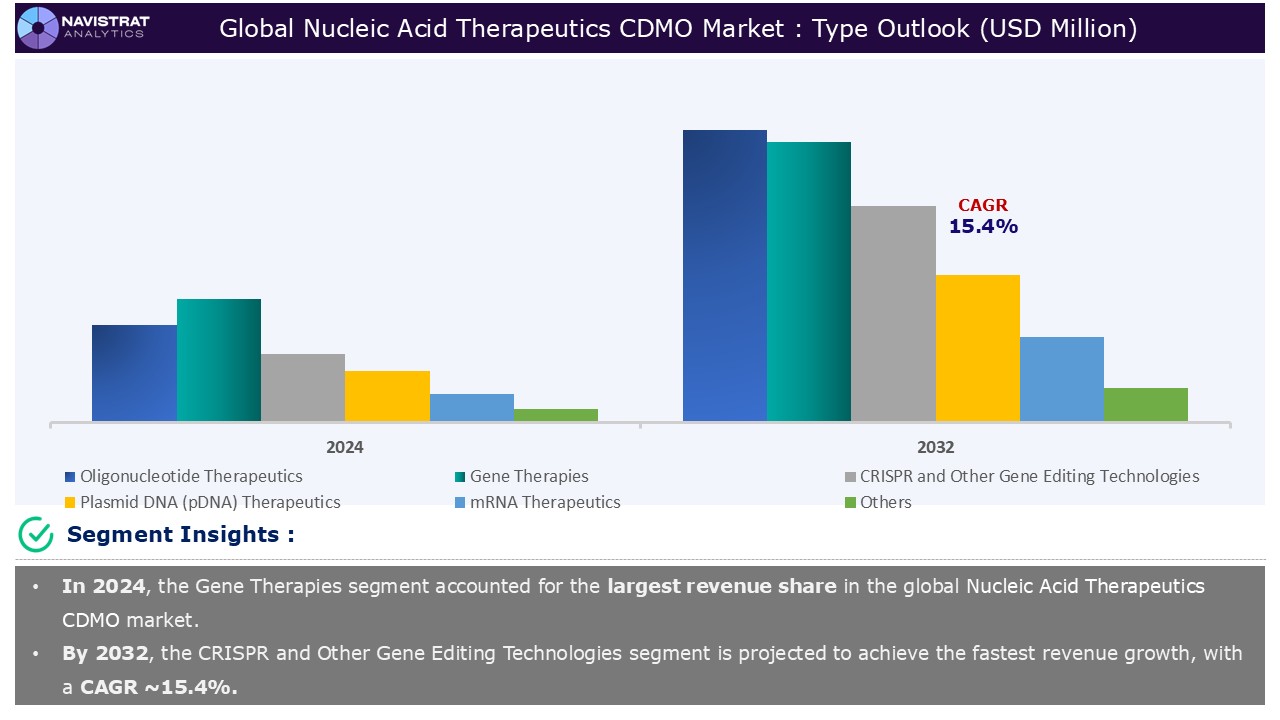
Type Segment Insights and Analysis:
Based on the type, the nucleic acid therapeutics CDMO market is segmented into oligonucleotide therapeutics, gene therapies, CRISPR and other gene editing technologies, plasmid DNA (pDNA) therapeutics, mRNA therapeutics, and others.
Gene therapies segment contributed the largest market share in 2024. The ability of small nucleic acids to influence gene expression through a variety of mechanisms has been extensively studied. Compared to traditional treatments, tiny nucleic acid medicines have the potential to achieve long-term or even curative effects through gene editing. Furthermore, the development of medications based on disease target genes has significantly broadened the applicability of nucleic acid treatments. The method of action and great specificity of nucleic acids indicate that they have significant potential in cancer treatment.
On January 2025, Evonik established a collaboration with ST Pharm, a manufacturer of active components for gene therapy, to broaden its RNA and nucleic acid therapeutic offerings. Through the collaboration, Evonik will be able to provide ST Pharm with tailored nucleic acids in addition to its lipid and lipid nanoparticle (LNP) medicinal product development capabilities. This streamlined strategy allows pharmaceutical companies to reduce complexity while increasing the speed to market for nucleic acid therapies.
CRISPR and other gene editing technologies is expected to register the fastest growth rate during the forecasted period. The CRISPR-Cas13 system has emerged as a game-changing tool for RNA editing, creating new potential for the creation of nucleic acid therapies. Its applications include inhibiting disease-causing genes, repairing splicing errors, and regulating immunological responses. This new method has great potential for the treatment of genetic problems. In diseases like sickle cell anaemia and muscular dystrophy, where specific genetic variants contribute to disease pathology, CRISPR-Cas9 gene editing provides an opportunity to fix these mistakes in the DNA.
On July 2024, Agilent Technologies Inc. stated that it has reached a definitive deal to purchase BIOVECTRA, a leading specialized contract development and manufacturing organization, for USD 925 million. The acquisition expands Agilent’s CDMO capabilities in oligonucleotides and CRISPR therapies. Both BIOVECTRA and Agilent are fully integrated CDMOs with cutting-edge facilities that adhere to current Good Manufacturing Practices (cGMP), a high standard for procedures, facilities, and controls utilized in the production, processing, and packaging of active medicinal ingredients.
Technology Segment Insights and Analysis:
Based on the technology, the nucleic acid therapeutics CDMO market is segmented into solid-phase synthesis, enzymatic synthesis, cell-free expression systems, viral vector-based manufacturing, non-viral vector manufacturing, and others.
Viral vector-based manufacturing segment contributed the largest market share in 2024. These modified forms of naturally occurring viruses, known as adenoviruses, adeno-associated viruses, or lentiviruses, are designed to be safe and efficient carriers of therapeutic genetic material into cells. The need for viral vectors used in gene treatments and vaccines is fast increasing, but the complexity and cost of manufacturing, particularly at large numbers to fulfil clinical and commercial needs, provide a significant barrier to patient access. Partnering with a CDMO that has scalable manufacturing capacity is critical for solving this challenge. Quality CDMOs have established ties with reputable suppliers, ensuring the availability of high-quality products.
On June 2024, ProBio Inc, a New Jersey-based contract development and manufacturing organization (CDMO), has expanded its plasmid DNA and viral vector production capabilities with the launch of a new cutting-edge facility in Hopewell, New Jersey. This cutting-edge facility will serve as the hub for North American operations, dramatically increasing ProBio’s ability to support the production of life-changing cell and gene treatments in North America. The Hopewell facility is approximately 128,000 square feet and includes office, laboratory, and manufacturing spaces outfitted with cutting-edge equipment and technology for process development.
Indication Segment Insights and Analysis:
Based on the indication, the nucleic acid therapeutics CDMO market is segmented into oncology, rare genetic disorders, infectious diseases, neurological disorders, and others.
Oncology segment contributed the largest market share in 2024. According to American Cancer Society, excluding non-melanoma skin cancer, at least 40% of newly diagnosed malignancies in US adults (811,000 cases in 2025) are theoretically avoidable. Cigarette smoking, excess body weight, and alcohol intake account for 19%, 8%, and 5%, respectively. In 2025, the US is expected to have 316,950 new cases of invasive breast cancer in women and 2,800 in men, as well as 59,080 cases of ductal carcinoma in situ (DCIS) in women. In 2025, a projected 42,680 people would die from breast cancer, with 42,170 women and 510 males.
NATs have the benefit of being simple to chemically synthesis into sequences and conjugate with a variety of functional moieties, ranging from small-molecule medicines to inorganic nanoparticles. Furthermore, NATs can be paired with other anti-cancer medicines, such as chemotherapy, radiation therapy, immunotherapy, and even other NATs, resulting in more effective anti-cancer outcomes.
On August 2024, AGC Biologics and NEC Bio Therapeutics have established a collaboration to accelerate the development of NECVAX-NEO1, an orally administered, bacteria-based DNA vaccine that targets patient-specific tumor neoantigens. This crucial and promising alliance intends to improve the production of tailored cancer therapies by combining both firms’ biotechnology strengths. New Phase 1/2 clinical trials for NECVAX-NEO1 are set to commence in cancer patients in 2024 and 2025. These trials will be critical in determining the efficacy and safety of this revolutionary medication, which could provide new hope to numerous cancer patients.
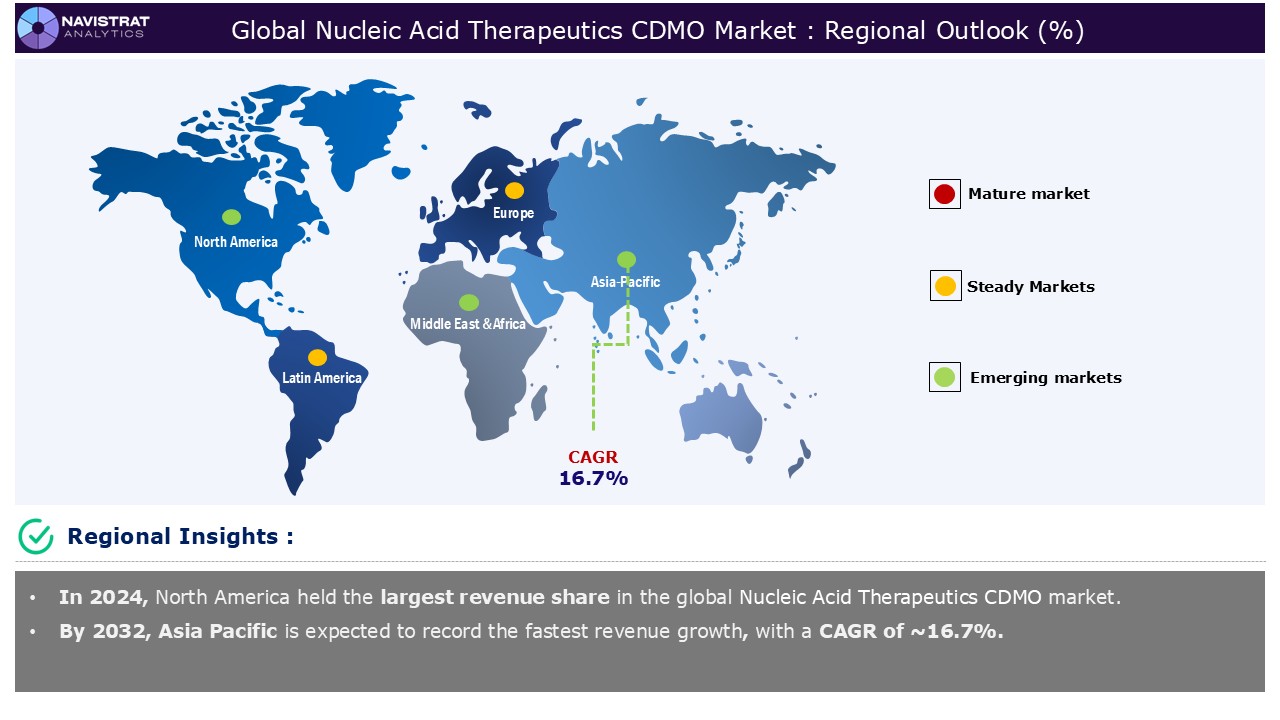
Geographical Outlook:
Nucleic acid therapeutics CDMO market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Nucleic Acid Therapeutics CDMO Market:
North America is registered to have highest market share in nucleic acid therapeutics CDMO market in 2024. This is mainly driven by increased demand for mRNA therapies, gene therapies, and CRISPR-based treatments. According to the US Centres for Disease Control and Prevention (CDC), haemophilia affects about one in every 5,617 live male births. In the United States, there are around 30,000-33,000 males with haemophilia. More than half of those diagnosed with haemophilia A have the severe variant. Haemophilia A is four times more frequent than haemophilia B.
On September 2024, TriLink BioTechnologies and Alphazyme, both part of Maravai LifeSciences, have teamed up to develop CleanScribe RNA Polymerase, a revolutionary enzyme that decreases double-stranded RNA (dsRNA) in mRNA generation. The novel product decreases dsRNA by up to 85%, enabling the development of safer, more effective mRNA therapies. CleanScribe RNA Polymerase is a new DNA-dependent RNA polymerase that catalyzes the in vitro transcription (IVT) of a recombinant gene controlled by the T7 promoter.
Asia Pacific Nucleic Acid Therapeutics CDMO Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period, driven by advancements in manufacturing technologies and increasing outsourcing to CDMOs & CMOs. China, India, and South Korea are expected to lead the Asia Pacific Pharmaceutical Contract Development and Manufacturing Organization (CDMO) market because of government backing, foreign investment incentives, and the rising prevalence of chronic diseases and aging populations. The use of new technologies like as AI, machine learning, and automation will improve CDMO services by reducing drug development time and increasing production.
On September 2024, AGC Inc., the parent company of AGC Biologics, and MEDINET announced the signing of a strategic collaboration agreement to assist build the cell therapy CDMO business in Japan by uniting the capabilities of these two industry leaders. In these circumstances, to accelerate the expansion of each company’s cell therapy CDMO business, AGC and MEDINET have agreed to exchange and train manufacturing and quality human resources from both companies, as well as to supplement MEDINET’s development and manufacturing capabilities with AGC Biologics’ global cell therapy sites.
Europe Nucleic Acid Therapeutics CDMO Market:
Europe is expected to have considerable market share in 2024 and leader in high-value CDMO services such as biologics, cancer APIs, and innovative drug formulations. European CDMOs are more focused than ever on transparency and long-term cooperation, resulting in greater alignment on quality standards and EU laws. This emphasis on quality distinguishes European CDMOs as capable partners in maintaining supply stability and regulatory compliance in a complicated global landscape.
On February 2025, TriLink BioTechnologies, a Maravai LifeSciences company and global provider of life science reagents and services, has partnered with Avantor, Inc., a leading global provider of mission-critical products and services to customers in the life sciences and advanced technology industries, to broaden the availability of its innovative nucleic acid products in Europe, the Middle East, and Africa (EMEA).
Competition Analysis:
The nucleic acid therapeutics CDMO market is characterized by a fragmented structure, with several players competing across various segments and regions. list of major players included in the nucleic acid therapeutics CDMO market report are:
- Danaher Corporation
- Merck KGaA
- Agilent Technologies, Inc
- Thermo Fisher Scientific
- AGC Biologics
- KNC Laboratories Co., Ltd.
- LGC Limited
- Wuxi AppTec
- Syngene International Limited
- BIOSPRING
- Univercells Inc.
- Exothera
- Asymchem Inc.
- Ajinomoto Co., Inc.
- Curia Global, Inc.
Strategic Developments in Nucleic Acid Therapeutics CDMO Market:
- In February 2025, Maravai LifeSciences, Inc., a global provider of life science reagents and services to researchers and biotech entrepreneurs, announced the successful completion of its previously announced acquisition of Officinae Bio’s DNA and RNA business. This acquisition brings together Officinae Bio’s AI-enabled mRNA design platforms with Maravai and TriLink BioTechnologies’ leading drug substance manufacturing capabilities, providing customers with comprehensive expertise and novel technologies for a quick, calculated progression through the mRNA sequence-optimization phase and into clinical testing and commercial manufacturing.
- In June 2024, WACKER has achieved a milestone by establishing an mRNA competency center at its biotech facility in Halle, Germany. The new facility enables large-scale manufacturing of active components derived from messenger ribonucleic acid (mRNA), such as anti-Covid mRNA vaccines. WACKER has invested over 100 million euros in this construction project. More than 100 highly qualified positions have already been created in Halle.
- In May 2024, Aldevron, a leading global manufacturer of DNA, RNA, and proteins used in cell and gene therapies, as well as vaccine development, has announced a strategic partnership with Acuitas Therapeutics, Inc., a private biotechnology company that specializes in developing lipid nanoparticle-based delivery systems for nucleic acid therapeutics.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Nucleic Acid Therapeutics CDMO Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 4,558.8 Million |
| Market Growth Rate in CAGR (2025–2032) | 13.5% |
| Market Revenue forecast to 2032 | USD 12,613.8 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Nucleic Acid Therapeutics CDMO market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Type Outlook (Revenue, USD Million; 2022-2032)
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Service Type Outlook (Revenue, USD Million; 2022-2032)
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Technology Outlook (Revenue, USD Million; 2022-2032)
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Indication Outlook (Revenue, USD Million; 2022-2032)
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Nucleic Acid Therapeutics CDMO market report
The market size of nucleic acid therapeutics CDMO market was 4,558.8 million in 2024.
The market size of nucleic acid therapeutics CDMO market is expected to register compound annual growth rate (CAGR) of 13.5% over the forecast period.
Rising demand for nucleic acid-based therapies, advancements in manufacturing technologies, and increasing outsourcing to CDMOs & CMOs are major key factors driving the market revenue growth of the nucleic acid therapeutics CDMO market.
High manufacturing costs and limited skilled workforce and infrastructure are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 16.7%.
Gene Therapies is the major leading segment of nucleic acid therapeutics CDMO market in terms of type.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Methods
- Primary
- Secondary
- Market Size Estimation
- Top-down Technology
- Bottom-up Technology
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Rising demand for nucleic acid-based therapies
- Advancements in manufacturing technologies
- Increasing outsourcing to CDMOs & CMOs
- Market Restraints
- High manufacturing costs
- Limited skilled workforce and infrastructure
- Market Opportunities
- Expansion of mRNA and gene editing therapies
- Rising demand for non-viral delivery technologies
- Government funding and investments
- Market Challenges
- Scalability and production bottlenecks
- Raw material supply chain issues
- Competition from in-house manufacturing
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Type Market Revenue Estimates and Forecasts, 2022-2032
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Service Type Market Revenue Estimates and Forecasts, 2022-2032
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Technology Market Revenue Estimates and Forecasts, 2022-2032
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Indication Market Revenue Estimates and Forecasts, 2022-2032
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
- North America
- North America Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- North America Nucleic Acid Therapeutics CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- North America Nucleic Acid Therapeutics CDMO Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- North America Nucleic Acid Therapeutics CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- North America Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- North America Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Europe
- Europe Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Europe Nucleic Acid Therapeutics CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Europe Nucleic Acid Therapeutics CDMO Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Europe Nucleic Acid Therapeutics CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Europe Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia-Pacific
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Asia-Pacific Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Latin America Nucleic Acid Therapeutics CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Latin America Nucleic Acid Therapeutics CDMO Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Latin America Nucleic Acid Therapeutics CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Latin America Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Latin America Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East & Africa
- North America
-
-
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oligonucleotide Therapeutics
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- MicroRNA (miRNA)
- Aptamers
- Gene Therapies
- DNA-based gene therapies
- RNA-based gene therapies
- CRISPR and Other Gene Editing Technologies
- CRISPR-Cas9
- Zinc Finger Nucleases (ZFNs)
- Transcription Activator-Like Effector Nucleases (TALENs)
- Plasmid DNA (pDNA) Therapeutics
- mRNA Therapeutics
- Others
- Oligonucleotide Therapeutics
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Process Development
- Manufacturing Services
- Analytical & Quality Control Services
- Others
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Solid-Phase Synthesis
- Enzymatic Synthesis
- Cell-Free Expression Systems
- Viral Vector-Based Manufacturing
- Non-Viral Vector Manufacturing
- Others
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Others
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Middle East & Africa Nucleic Acid Therapeutics CDMO Market By Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
-
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- Danaher Corporation
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Merck KGaA
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Agilent Technologies, Inc
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Thermo Fisher Scientific
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- AGC Biologics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- KNC Laboratories Co., Ltd.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- LGC Limited
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Wuxi AppTec
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Syngene International Limited
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- BIOSPRING
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Univercells Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Exothera
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Asymchem Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Ajinomoto Co., Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Curia Global, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

