Small Interfering RNAs (siRNAs) Therapeutics Market Overview and Key Insights:
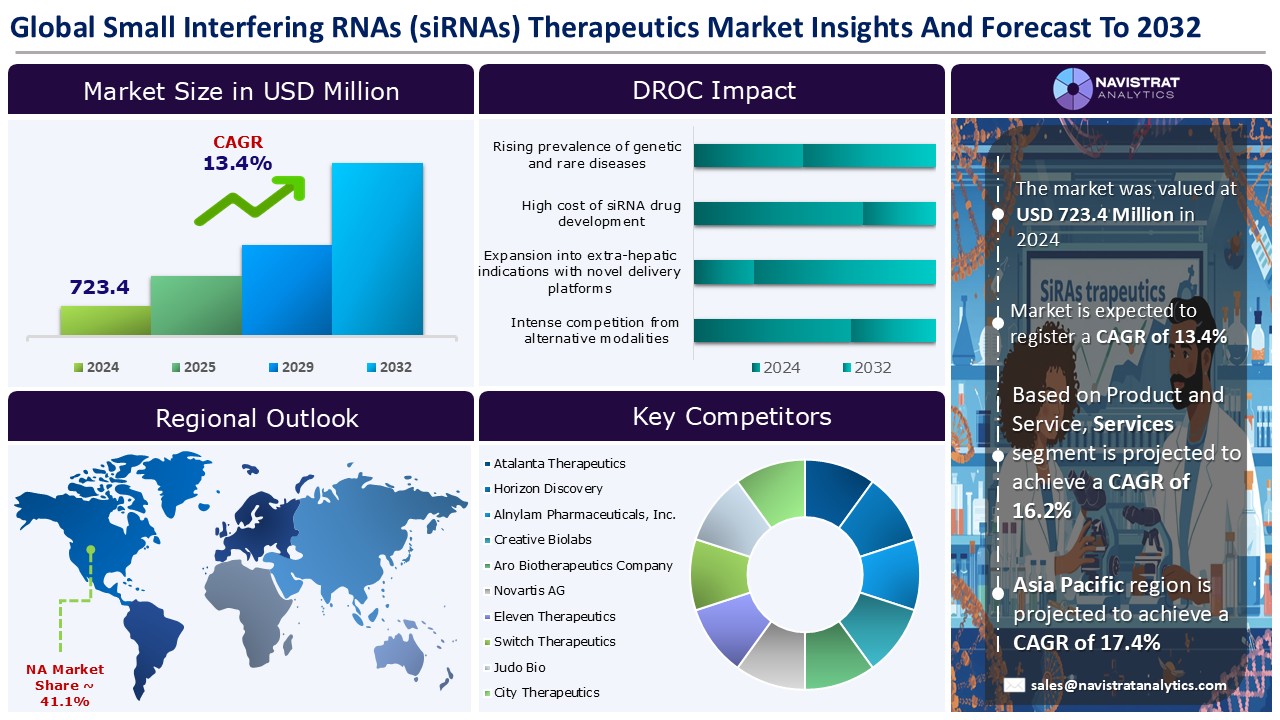
The Small Interfering RNAs (siRNAs) therapeutics market size reached USD 723.4 million in 2024 and is expected to register a revenue CAGR of 13.4% during the forecast period. Small interfering RNAs (siRNAs) are typically small antisense RNAs of 20-25 nucleotides in length. They are derived from double-stranded RNAs and play an important role in RNA interference pathways through post-transcriptional regulation of gene expression.
Market Drivers:
Rising prevalence of genetic and rare diseases is a key driver of revenue growth in the Small Interfering RNAs (siRNAs) therapeutics market. According to the American Cancer Society, pancreatic cancer is expected to affect around 67,440 individuals (34,950 men and 32,490 women) in the United States in 2025, with 51,980 people (27,050 men and 24,930 women) dying from the disease. Pancreatic cancer has an average lifetime risk of one in 56 males and one in 60 women.
Pancreatic Ductal Adenocarcinoma (PDAC) is the most lethal type of pancreatic cancer, accounting for more than 90% of all pancreatic malignancies. There were 62,210 new cases reported in the United States in 2023, compared to 49,380 deaths in 2022. Of the 57,600 cases reported in 2020, 55% had advanced to metastatic illness. PDAC is responsible for 2% of all cancer diagnoses and 5% of all cancer deaths in the United States, emphasizing the essential need for earlier identification.
Recent improvements in drug delivery technologies show significant promise for improving siRNA-based therapies and creating a new class of pharmaceuticals known as nano-siRNA medications. siRNA has an inherent advantage over small molecular therapeutics and monoclonal antibody drugs because it performs its function through complete Watson-Crick base pairing with mRNA, whereas small molecule and monoclonal antibody drugs must recognize the complex spatial conformation of specific proteins.
On March 2025, Alnylam Pharmaceuticals, Inc., the leading RNAi therapeutics company, announced that the U.S. Food and Drug Administration (FDA) has approved Qfitlia (fitusiran), the sixth Alnylam-discovered RNAi therapeutic approved in the United States and the first and only therapeutic to lower antithrombin (AT), a protein that inhibits blood clotting, with the goal of promoting thrombin generation to rebalance hemostasis and prevent bleeds. Qfitlia has the potential to help the estimated one million persons living with hemophilia A and B worldwide.
Market Opportunity:
Expansion into extra-hepatic indications with novel delivery platforms act as an opportunity for Small Interfering RNAs (siRNAs) therapeutics market. Targeted oligonucleotide delivery to liver hepatocytes via N-acetylgalactosamine (GalNAc) conjugates that bind to the asialoglycoprotein receptor has emerged as a game-changing method in the therapeutic oligonucleotide sector. This technology has resulted in the licensure of givosiran for the treatment of acute hepatic porphyria, with additional seven conjugates undergoing registrational review or phase 3 trials, as well as at least 21 conjugates in previous stages of clinical research.
Unlike the liver, there are no FDA-approved extrahepatic treatments for systemically injected siRNA, including malignancies. Most nanoparticles, including polyplexes, rely on the EPR effect, which typically leads to unreliable and variable nanoparticle administration, with few clinical trial successes. Considerable attempts for extrahepatic disorders have resulted in few achievements, all of which have used chemotherapeutic drugs rather than nucleic acids, including siRNA.
Recent Trends:
Emerging trends include GalNAc conjugation for liver-targeted delivery, expanding delivery beyond the liver, advanced nanoparticles, and stimuli-responsive systems, and machine learning–augmented design.
The introduction of machine learning and its subsequent incorporation into small interfering RNA (siRNA) research ushers in a new era in the field of RNA interference. Despite hurdles such as the requirement for big, high-quality datasets and the complexities of biological systems, the continuing development of powerful machine learning models and feature engineering techniques provides a promising view for the field’s future.
The use of machine learning, ranging from classical algorithms to deep learning methods, is becoming increasingly important in siRNA research, including structural profiling, vsiRNA prediction, cellular uptake prediction, and mRNA cleavage site identification. Although the methodology and applications differ, the overall progress in the subject demonstrates machine learning’s transformative potential in this domain.
Restraints & Challenges:
Small interfering RNA (siRNA) offers a promising approach for gene silencing by targeting specific genes. While several siRNA-based therapies have already gained approval from the U.S. Food and Drug Administration (FDA), their broader clinical application is hindered by multiple biological obstacles. However, because siRNA molecules are hydrophilic and negatively charged, transporting them to target cells presents a substantial problem. This makes it difficult for them to passively diffuse across the plasma membrane’s hydrophobic lipid bilayer. Furthermore, the size, charge, and hydrophilicity of siRNA molecules provide impediments to their cellular uptake since they reject negatively charged phospholipids in the plasma membrane and are larger than most membrane channels and transporters.
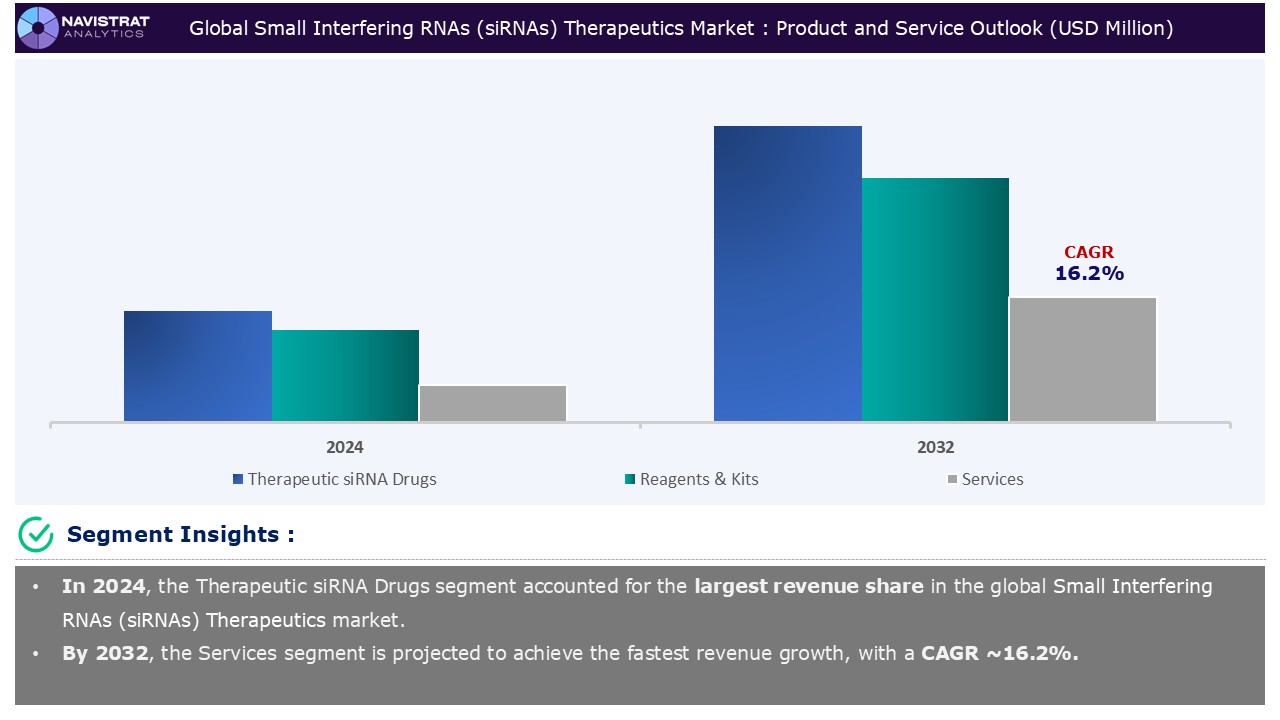
Product and Service Segment Insights and Analysis:
Based on the product and service, the Small Interfering RNAs (siRNAs) therapeutics market is segmented into therapeutic siRNA Drugs, reagents & kits, and services.
Therapeutic siRNA drugs segment contributed the highest market share in 2024. A new and expanding class of RNA therapies has been made possible by the clinical use of RNA interference (RNAi) biological mechanisms. These treatments work by regulating the expression of target genes at the posttranscriptional level. More RNAi therapies are being developed as a result of the increasing number and broadening illness indications of authorized siRNA medicines. The most frequent therapeutic approvals for rare disorders can be partially explained by the fact that siRNA oligonucleotides can be manufactured with reduced research and development expenditures because the mRNA sequences for their targets are frequently known.
On November 2024, Arrowhead Pharmaceuticals, Inc. and Sarepta Therapeutics, Inc., the pioneer in precision genetic treatment for rare diseases, have signed an exclusive worldwide licensing and cooperation agreement. Sarepta will acquire the only worldwide rights to several clinical, preclinical, and discovery-stage initiatives for uncommon, hereditary lung, muscular, and central nervous system (CNS) disorders. The clinical projects make use of Arrowhead’s own Targeted RNAi Molecule (TRiM) technology, which is made to deliver siRNA to various bodily tissues and cell types to start the RNA interference mechanism and cause target gene knockdown that is both quick and long-lasting.
Delivery Modality Segment Insights and Analysis:
Based on the delivery modality, the Small Interfering RNAs (siRNAs) therapeutics market is segmented into conjugate-based delivery, nanoparticle-based delivery, biological carriers, and others.
Nanoparticle-based delivery segment contributed the largest market share in 2024. The delivery of siRNA has benefited greatly from nanotechnology over the past few decades due to the inherent benefits of NP-based delivery systems, including low toxicity, low immunogenicity, biocompatibility, high encapsulation, controlled release, ease of modification, and targeting property based on the enhanced permeability and retention (EPR) effect.
Lipid-based NPs, such as lipopolyplex (LPR), lipid nanoparticles (LNPs), and cationic liposomes, have been widely employed in drug delivery. The most popular carrier for siRNA delivery among them is cationic liposomes. These elements are designed to effectively transport siRNA to specific cells and silence genes. Because ionizable lipids’ charge depends on the pH of their surroundings, research has indicated that they are less harmful than cationic lipids.
On April 2023, researchers from the Universities of Amsterdam and Leiden have collaborated to create specific molecular nanocages for the transport of siRNA. The tiny chemical building blocks, known as ditopic ligands, that make up the nanocages are joined by metal atoms. Because a typical cage has 12 metal atoms and 24 ligands, the acronym M12L24 was created. To create molecular cages with varying siRNA binding affinities, the researchers created and synthesized five distinct ligands.
Application Segment Insights and Analysis:
Based on the application, the Small Interfering RNAs (siRNAs) Therapeutics market is segmented into therapeutics and research.
Therapeutics diseases segment contributed the largest market share in 2024. It is further segmented into oncology, genetic & rare diseases, metabolic & endocrine disorders, respiratory diseases, ophthalmology, CNS disorders, and others. According to the National Institute of Health, the prevalence of Huntington disease, a rare neurological illness, is 2.7 per 100,000 people globally. The frequency among populations of Caucasian descent is considerable, ranging from 10.6 to 13.7 per 100,000. For Asian and African countries, the prevalence is far lower. Geographically, the incidence ranges by more than ten times. About one out of every 10,000 to 20,000 persons in the US has Huntington’s disease. It transcends all racial and ethnic divides and has an equal impact on men and women. Symptoms usually start to appear between the ages of 30 and 55.
On January 2025, Atalanta Therapeutics, a biotechnology company that is pioneering RNA interference (RNAi) for the treatment of neurological diseases, announced today that it has completed a USD 97 million Series B financing to support Phase 1 clinical trials of its investigational RNAi therapies for KCNT1-related epilepsy and Huntington’s disease. This financing confirms the transformational potential of Atalanta’s best-in-class di-siRNA platform for delivering oligonucleotide treatments to the central nervous system.
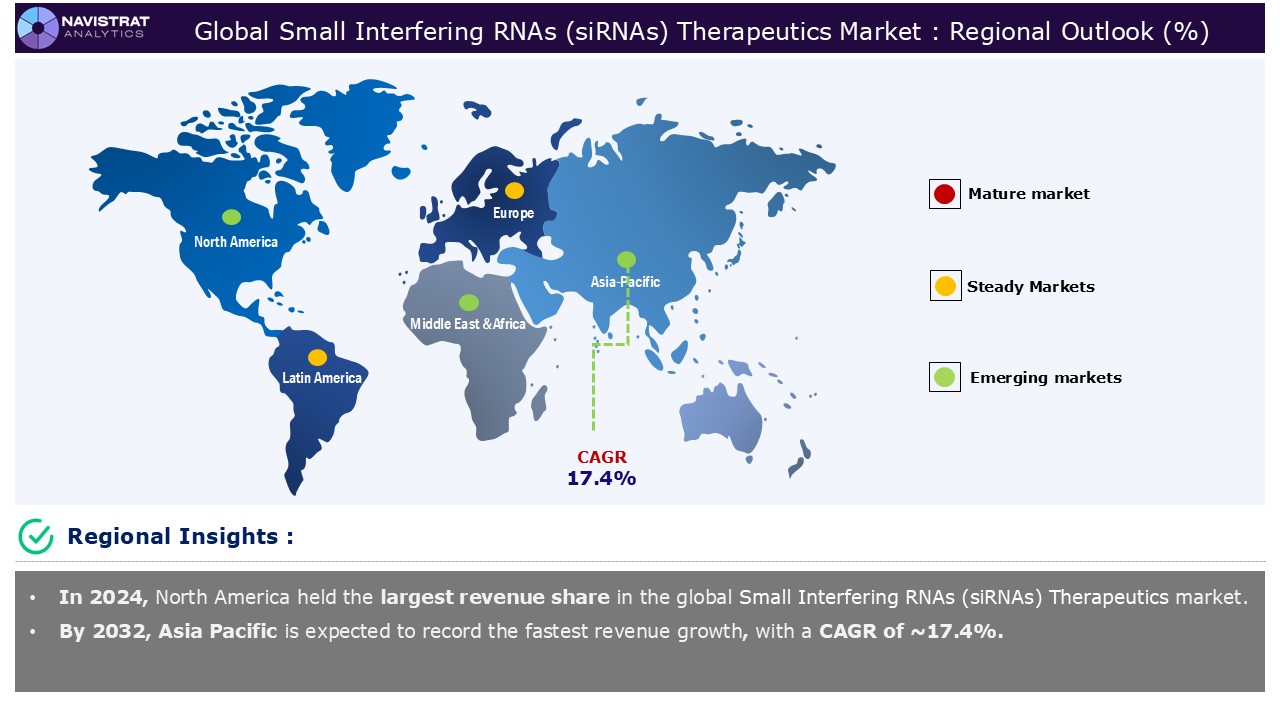
Geographical Outlook:
Small Interfering RNAs (siRNAs) therapeutics market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Small Interfering RNAs (siRNAs) Therapeutics Market:
North America is registered to have highest market share in Small Interfering RNAs (siRNAs) therapeutics market in 2024. This is mainly driven by rising prevalence of genetic and rare diseases, advancements in RNA delivery technologies improving therapeutic efficacy, and increasing pipeline of siRNA-based drugs with regulatory approvals. According to the Center of Disease Control and Prevention (CDC), the prevalence of hemophilia in the United States is unknown, however estimates indicate that approximately 33,000 people assigned male at birth live with hemophilia nationally. The illness primarily affects people who were assigned male at birth. More than half of those diagnosed with hemophilia A have the severe variant. Hemophilia A is four times more frequent than hemophilia B. Hemophilia affects all races and ethnicities.
On May 2025, AbbVie and ADARx Pharmaceuticals, a late-stage biotechnology company developing next-generation RNA therapeutics, have announced a collaboration and license option agreement to develop small interfering RNA (siRNA) therapies for a variety of diseases, including neuroscience, immunology, and oncology. The strategic alliance will take use of ADARx’s RNA discovery capabilities and patented siRNA technology, which has the potential to provide long-term and precise mRNA silencing. AbbVie will offer expertise in antibody engineering, antibody drug conjugates (ADCs), and tissue delivery techniques as needed to ADARx’s research initiatives.
Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period, augmented by rising prevalence of genetic and rare diseases and increasing pipeline of siRNA-based drugs with regulatory approvals. Rapid growth in the biotechnology sector, combined with investment in research, particularly in China, Japan, and India, is driving the spread of siRNA treatments in Asia-Pacific. Furthermore, the growing prevalence of diseases and genetic problems creates a need for novel treatments. As the industry evolves rapidly, optimizing medication development through personalized approaches is critical. Researchers are developing several kinds of siRNA medicines, each using a unique delivery technology and strategy, to target extrahepatic tissues.
On April 2025, Rona Therapeutics, a biotechnology company pioneering innovative RNA-targeted therapies, announced that China’s National Medical Products Administration (NMPA) has approved its Investigational New Drug (IND) application for RN1871, a small interfering RNA (siRNA) drug targeting angiotensinogen (AGT). RN1871 targets AGT mRNA expression in the liver by directly suppressing the development of a critical precursor protein in the Renin-Angiotensin-Aldosterone System (RAAS). The RAAS pathway regulates blood pressure, and its overactivation drives hypertension progression.
Europe Small Interfering RNAs (siRNAs) Therapeutics Market:
Europe is expected to have considerable market share in 2024. Since its discovery, the use of siRNA in research and therapy has advanced dramatically, with continued development aimed at solving problems such as efficiently transporting siRNA molecules into cells and specifically targeting disease-causing genes while avoiding off-target effects. The success of siRNA technology in the lab has led to the creation of siRNA-based therapies. These are now beginning to be approved for clinical use in treating disorders having genetic components. Recent developments, such as the approval of new siRNA-based medications and advancements in delivery techniques, indicate a bright future for gene-targeted therapeutics.
On June 2025, Crucible Therapeutics, a biotechnology spinout from the University of Sheffield, has been given GBP 2.3 million to find breakthrough medicines to address the fundamental causes of motor neuron disease (MND). The investment from the Innovate UK Biomedical Catalyst initiative will help Crucible improve its tailored siRNA approach for treating MND, also known as amyotrophic lateral sclerosis (ALS), and other debilitating neurodegenerative disorders.
Competition Analysis:
The Small Interfering RNAs (siRNAs) therapeutics market is characterized by a fragmented structure, with several players competing across various segments and regions. List of major players included in the Small Interfering RNAs (siRNAs) therapeutics market report are:
- Atalanta Therapeutics
- Horizon Discovery
- Alnylam Pharmaceuticals, Inc.
- Creative Biolabs
- Aro Biotherapeutics Company
- Novartis AG
- Eleven Therapeutics
- Switch Therapeutics
- Judo Bio
- City Therapeutics
- Sirius Therapeutics
- Rona Therapeutics
Strategic Developments in Small Interfering RNAs (siRNAs) Therapeutics Market:
- In May 2025, CRISPR Therapeutics, a biopharmaceutical company focused on developing transformative gene-based medicines for serious diseases, and Sirius Therapeutics, a clinical stage biotech company developing innovative small interfering RNA (siRNA) therapies for global markets, announced a strategic partnership today to develop and commercialize siRNA therapies. SRSD107 is a next-generation, long-acting siRNA that specifically inhibits Factor XI (FXI), a critical cause of pathological thrombosis, with minimal effect on normal hemostasis.
- In May 2025, Sirius Therapeutics announced the successful completion of roughly USD 50 million in Series B2 financing to further clinical development of the Company’s innovative siRNA therapies for cardiometabolic illnesses, as well as the continuous innovation of its next-generation RNA delivery technology. The financing round was led by a famous corporate venture capital firm and included a new investment, BioTrack Capital, as well as current investors OrbiMed, Creacion Ventures, and Hankang Capital.
- In July 2024, Sirnaomics Ltd., a leading biopharmaceutical company focused on the discovery and development of advanced RNAi therapeutics, announced that it has completed IND-enabling studies for STP125G, a siRNA therapeutic targeting Apolipoprotein C3 (ApoC3) using its proprietary GalAhead mxRNA technology. The safety and efficacy findings from the non-human primate (NHP) tests clearly support an IND submission with the US FDA to begin Phase I clinical research of STP125G for cardiovascular disease indications.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Small Interfering RNAs (siRNAs) Therapeutics Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 723.4 Million |
| Market Growth Rate in CAGR (2025–2032) | 13.4% |
| Market Revenue forecast to 2032 | USD 1,987.5 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Small Interfering RNAs (siRNAs) Therapeutics market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Product and Service Outlook (Revenue, USD Million; 2022-2032)
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Delivery Modality Outlook (Revenue, USD Million; 2022-2032)
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Application Outlook (Revenue, USD Million; 2022-2032)
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- End-Use Outlook (Revenue, USD Million; 2022-2032)
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Small Interfering RNAs (siRNAs) Therapeutics market report
The market size of Small Interfering RNAs (siRNAs) therapeutics market was 723.4 million in 2024.
The market size of Small Interfering RNAs (siRNAs) therapeutics market is expected to register compound annual growth rate (CAGR) of 13.4% over the forecast period.
Rising prevalence of genetic and rare diseases, advancements in RNA delivery technologies improving therapeutic efficacy, and increasing pipeline of siRNA-based drugs with regulatory approvals are major key factors driving the market revenue growth of the Small Interfering RNAs (siRNAs) therapeutics market.
High cost of siRNA drug development and delivery challenges for non-hepatic tissues limiting broader clinical application are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 17.4%.
Therapeutic siRNA drugs is the major leading segment of Small Interfering RNAs (siRNAs) therapeutics market in terms of product and service.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Methods
- Primary
- Secondary
- Market Size Estimation
- Top-down Delivery Modality
- Bottom-up Delivery Modality
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Rising prevalence of genetic and rare diseases
- Advancements in RNA delivery technologies improving therapeutic efficacy
- Increasing pipeline of siRNA-based drugs with regulatory approvals
- Market Restraints
- High cost of siRNA drug development
- Delivery challenges for non-hepatic tissues limiting broader clinical application
- Market Opportunities
- Expansion into extra-hepatic Applications with novel delivery platforms
- Growing potential in personalized medicine and rare/orphan diseases.
- Increasing interest in combination therapies with other modalities
- Market Challenges
- Intense competition from alternative modalities
- Intellectual property complexities with overlapping RNAi patents
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Product and Service Market Revenue Estimates and Forecasts, 2022-2032
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Delivery Modality Market Revenue Estimates and Forecasts, 2022-2032
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Application Market Revenue Estimates and Forecasts, 2022-2032
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- End-Use Market Revenue Estimates and Forecasts, 2022-2032
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
- North America
- North America Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- North America Small Interfering RNAs (siRNAs) Therapeutics Market By Delivery Modality, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- North America Small Interfering RNAs (siRNAs) Therapeutics Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- North America Small Interfering RNAs (siRNAs) Therapeutics Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- North America Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- North America Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Europe
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market By Delivery Modality, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia Pacific
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market By Delivery Modality, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia Pacific
- Asia Pacific Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market By Delivery Modality, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Latin America Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East and Africa
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutic siRNA Drugs
- Reagents & Kits
- Custom siRNA synthesis
- Knockdown assay kits
- Transfection reagents
- Services
- siRNA design & sequence optimization
- Bioinformatics/off-target prediction
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market By Delivery Modality, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Conjugate-based delivery
- Nanoparticle-based delivery
- Biological carriers
- Others
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Therapeutics
- Oncology
- Genetic & Rare Diseases
- Metabolic & Endocrine Disorders
- Respiratory Diseases
- Ophthalmology
- CNS Disorders
- Others
- Research
- Functional genomics
- Target validation
- High-throughput screening
- Cell-based assays
- Therapeutics
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Hospitals and Clinics
- Pharmaceutical and Biotechnology Companies
- Research Institutes and Academic Centers
- Contract Research Organizations (CROs)
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East and Africa
- Middle East & Africa Small Interfering RNAs (siRNAs) Therapeutics Market By Product and Service, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- North America
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- Atalanta Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Horizon Discovery
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Alnylam Pharmaceuticals, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Creative Biolabs
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Aro Biotherapeutics Company
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Novartis AG
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Eleven Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Switch Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Judo Bio
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- City Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sirius Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Rona Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

