Global Adeno Associated Virus (AAV) Vector Manufacturing Market Overview and Key Insights:
The global Adeno Associated Virus (AAV) Vector Manufacturing market size reached USD 1,148.4 Million in 2024 and is expected to register a revenue CAGR of 19.6% during the forecast period. Adeno-associated virus (AAV) is a flexible viral vector technology that can be programmed for highly specialized functionality in gene therapy applications. AAV’s unique biological and physicochemical features make it suitable for a wide range of clinical applications in a variety of disorders.

Market Drivers:
Increase in prevalence of chronic disorders is a key driver of revenue growth in the Adeno Associated Virus (AAV) vector manufacturing market. According to the US Centers for Disease Control and Prevention (CDC), hemophilia affects about one in every 5,617 live male births. In the United States, there are around 30,000-33,000 males with hemophilia. More than half of those diagnosed with hemophilia A have the severe variant. Hemophilia A is four times more frequent than hemophilia B. Hemophilia affects all races and ethnicities. Furthermore, nearly three-quarters of men with hemophilia had a history of hepatitis C virus (HCV) infection, and around one-fourth had a history of human immunodeficiency virus (HIV) infection.
Technological advancements in AAV manufacturing are driving the demand for Adeno Associated Virus (AAV) vector manufacturing market. The landscape of AAV manufacturing has experienced a wave of technological improvements aimed at increasing vector production efficiency, specificity, and purity. One of the most significant breakthroughs is enhanced efficiency in AAV vector generation. On October 2024, Forge Biologics, a member of Ajinomoto Bio-Pharma Services and a leading manufacturer of genetic medicines, has announced the release of its FUEL manufacturing platform, which will provide AAV gene therapy developers with a more efficient and accelerated foundation for manufacturing as they advance new programs and target a broader range of diseases. The FUEL (Foundation for Unleashing Excellence in Life-Changing Therapies) platform includes several new technological advancements, including Forge’s pEMBR 2.0 Ad helper plasmid, which is one of the smallest commercially available at 8.9kb and offers an improved safety profile and manufacturing efficiency.
Market Opportunity:
Increasing focus on rare diseases acts as an opportunity for the Adeno Associated Virus (AAV) vector manufacturing market. Stargardt Disease, the most common kind of hereditary macular degeneration, affects around 30,000 persons in the United States. Stargardt disease causes gradual vision loss due to the degradation of photoreceptor cells in the macula, the center region of the retina.
On October 2024, Complement Therapeutics, a preclinical stage biotechnology company creating novel therapeutics for complement-mediated diseases; Pharmaron Biologics (UK) Ltd, a strategic partner for gene therapy developers; and The Cell and Gene Therapy Catapult (CGT Catapult), an independent innovation and technology organization specializing in the cell and gene therapy industry, have been granted a GBP 1.4m Innovate UK Transforming Medicines Manufacturing (TMM) Programme grant to develop a perfusion process that will enhance gene therapy production.
Recent Trends:
Recent trends include the high-throughput screening and transcriptomics integration, Artificial Intelligence (AI) in AAV production, and vector delivery systems.
Integrating artificial intelligence (AI) and machine learning (ML) into AAV manufacturing is another promising developing concept. AI and ML technology can improve numerous parts of the manufacturing process, including as predicting yield results and spotting possible problems before they occur. These tools can evaluate massive datasets and deliver insights that improve industrial efficiency and reliability.
On September 2024, Asimov, a synthetic biology business that advances therapeutic design and production, has launched the AAV Edge System, a comprehensive set of tools for designing and producing adeno-associated viral (AAV) gene therapies. The system provides gene therapy scientists with a single point of access to a suite of best-in-class technologies designed to accelerate gene therapy development.
Restraints & Challenges:
Limited manufacturing capacity poses a significant hurdle to the implementation of Adeno Associated Virus (AAV) vector manufacturing market. Because of its outstanding safety profile and effective transduction to diverse target tissues, adeno-associated virus (AAV) has emerged as a key platform for gene delivery in the treatment of a variety of disorders. However, large-scale synthesis and long-term storage of viral vectors is inefficient, resulting in lower yields, moderate purity, and a shorter shelf life than recombinant protein therapies. Scaling up involves complexity such as consistency, quality control, and regulatory compliance. One of the most difficult difficulties is ensuring that the production process is consistent and scalable throughout tiny laboratory batches to large-scale clinical productions.
Scale of Operations Segment Insights and Analysis:
Based on the scale of operations, the Adeno Associated Virus (AAV) vector manufacturing market is segmented into clinical, preclinical, and commercial.
Clinical segment contributed largest share in 2024. Adeno-associated virus (AAV) has emerged as a pivotal delivery tool in clinical gene therapy owing to its minimal pathogenicity and ability to establish long-term gene expression in different tissues. Recombinant AAV (rAAV) has been engineered for enhanced specificity and developed as a tool for treating various diseases.
On November 2024, Affinia Therapeutics, a pioneering gene therapy company focused on developing adeno-associated virus (AAV)-based treatments for severe cardiovascular and neurological conditions, has partnered with Forge Biologics, a prominent genetic medicine manufacturer and part of the Ajinomoto Bio-Pharma Services group. This collaboration involves the technology transfer and cGMP-compliant production of clinical trial materials to advance Affinia’s development programs. The agreement includes Affinia’s investigational therapy, AFTX-201, targeting BAG3 dilated cardiomyopathy—a severe monogenic heart condition that affects over 70,000 patients across the U.S., Europe, and the U.K.
Commercial segment is expected to have the highest growth rate throughout the forecasted period. Recombinant AAVs have acquired favor in the gene therapy area because to their well-established safety profile, broad and configurable tropism, no pathogenicity, and capacity to achieve long-term expression in non-dividing cells with a single treatment. Furthermore, with capsid engineering, the tropism of these vectors can be increased to boost the efficacy of transduction of the desired tissue type, minimize the host immune response, and de-target from unwanted tissues to improve safety.
On March 2024, Agathos Biologics, a leading biotechnology business in genetic medicine, has announced the debut of a recombinant adeno-associated virus (rAAV) production service based on the company’s innovative proprietary cell line, AE1-BHK. The company made its first sale of rAAV to Genovac, a contract research and manufacturing organization (CRO/CMO) that discovers, develops, and produces antibodies for the therapeutic, diagnostic, and research markets. Agathos used a well-established method known as triple transfection to create rAAV with a transgene sequence given by Genovac in AE1-BHK.
Application Segment Insights and Analysis:
Based on the application, the Adeno Associated Virus (AAV) vector manufacturing market is segmented into gene therapy, vaccine, and cell therapy.
Gene therapy segment contributed largest market revenue share in 2024. Adeno-associated virus (AAV) vectors have emerged as the most popular choice in clinical trials and FDA-approved applications. This is due to their broad tissue tropism, relatively low risk profile, and adaptable manufacturing procedures. Importantly, AAV is not harmful, seldom integrates into the host DNA, and can maintain long-term transgenic expression. Furthermore, vectors based on certain AAV serotypes have an intrinsic ability to efficiently enter cells and express transgenes, which improves transduction efficacy.
On April 2024, Latus Bio, Inc., a biotechnology firm exploring innovative gene therapy candidates for central nervous system (CNS) illnesses, has announced its launch and an initial Series A funding round of USD 54 million. The fundraising round is headed by 8VC and DCVC Bio. Latus is developing CNS gene therapy candidates using unique technologies previously utilized to detect and construct novel adeno-associated virus (AAV) capsid variations, with the goal of demonstrating exceptional potency, specificity, and safety.
Vaccine segment is expected to have the highest growth rate throughout the forecasted period. The vector genetic construct includes the lateral inverted terminal repeat (ITR), the only cis-acting element necessary for AAV DNA replication and encapsidation. Recombinant adeno-associated viruses (rAAV) are modified to become replication-defective entities capable of just infecting cells and delivering DNA to the nucleus.
On October 2023, ReiThera, an Italian contract development and manufacturing company, has extended its viral production operations facility in Castel Romano Technopole, Italy, in response to global demand for viral vectors. The Italian Medicines Agency (AIFA) has granted ReiThera operating permission to begin large-scale viral vector production for vaccines and gene therapy at the new production facility.

Purification Segment Insights and Analysis:
Based on the purification, the Adeno Associated Virus (AAV) vector manufacturing market is segmented into chromatography and ultrafiltration.
Chromatography segment contributed largest market revenue share and highest growth rate in 2024. Chromatography is an important aspect of the AAV manufacturing process. Chromatographic technologies offer the potential to allow for purification at larger scales and at a cheaper cost, but they frequently require serotype-specific tuning. Typically, two chromatographic stages are performed. A capture step to remove the bulk of the contaminants including host cell proteins (HCP) and DNA, followed by an anion exchange step to further reduce those contaminants, HCP and DNA, along with a reduction in the number of empty capsids that do not contain the therapeutic DNA payload.
On April 2024, ChromaTan, a bioprocessing and biotools advanced biomanufacturing technology platform development company, announced a partnership with Batavia Biosciences, a leading contract development and manufacturing organization, to develop and integrate ChromaTan’s BioRMB continuous counter-current elution technology into Batavia’s AAV HIP-Vax manufacturing platform. This initiative demonstrates ChromaTan and Batavia’s dedication to improving AAV manufacturing while lowering manufacturing costs and decreasing the environmental footprint of biopharmaceuticals through process intensification.
Geographical Outlook:
Adeno Associated Virus (AAV) vector manufacturing is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Adeno Associated Virus (AAV) Vector Manufacturing Market:
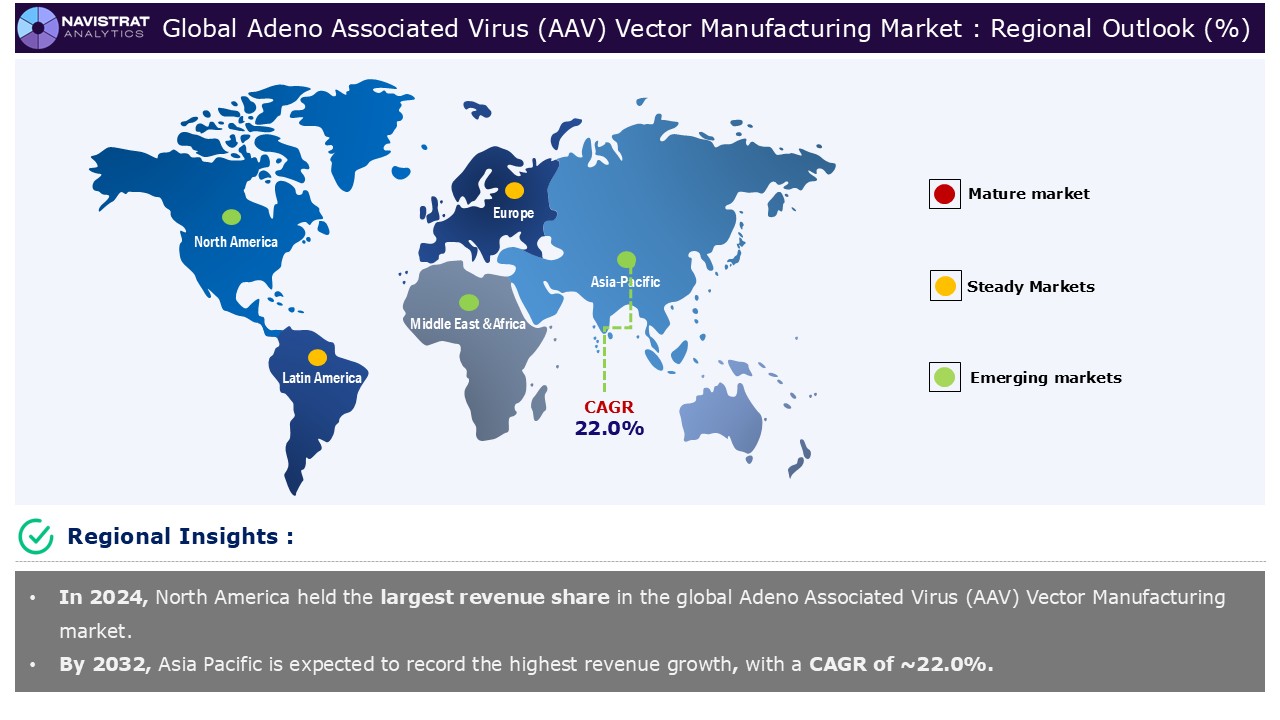
North America is registered highest market share in Adeno Associated Virus (AAV) vector manufacturing market in 2024 driven by increasing prevalence of chronic disorders and rising demand for gene therapy. According to the Centre of Disease Control and Prevention (CDC), every year in the United States, around 47,984 new cancer cases are discovered in areas of the body where human papillomavirus (HPV) is often prevalent. HPV causes around 37,800 of these malignancies. Cervical cancer is the most frequent HPV-related cancer in women, while oropharyngeal cancers are the most common in men.
On August 2024, Andelyn Biosciences, Inc., a leading and patient-focused contract development, and manufacturing organization (CDMO), has signed a license agreement with the Broad Institute to expand its AAV Curator Platform offering to include MyoAAV plasmids. The License Agreement authorizes Andelyn to use the MyoAAV plasmids to conduct research and development services for its clients developing gene treatments, including screening promising candidates at Andelyn and doing scale-up and pre-clinical development work in preparation for IND-enabling studies.
Asia Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period. The high incidence of chronic disorders, increase in demand for cell and gene therapy in the region are the main drivers driving the revenue in the region. Strategic collaborations are formed between biopharma businesses to improve research capacity, develop novel bioprocessing technologies, and promote industry expansion to accelerate drug development and commercialization. On July 2024, Merck, a prominent science and technology firm, has signed a non-binding Memorandum of Understanding (MoU) with Gene Therapy Research Institution Co., Ltd. (GTRI), a Japanese clinical-stage biotech start-up that specializes in gene therapy with adeno-associated virus (AAV) vectors. GTRI will produce its viral vector-based gene therapy for Parkinson’s Disease in accordance with Good Manufacturing Practices (GMP) using Merck’s Sf-RVN Insect Cell Line platform.
Europe Adeno Associated Virus (AAV) Vector Manufacturing Market:
Europe is to register a considerable market share in Adeno Associated Virus (AAV) vector manufacturing market in 2024.The region growth is primarily due to the increase in government investments and initiatives and for presence of innovative and robust leading companies. On April 2024, Ascend Advanced Therapies (Ascend), an end-to-end gene therapy development partner, has purchased Beacon Therapeutics’ Chemistry, Manufacturing, and Controls (CMC) team and Alachua, Florida headquarters. The acquisition adds an operational good manufacturing practice (GMP) facility, process and analytical development capabilities, and new specialists to the Ascend team. It also involves a long-term collaboration with Beacon to continue manufacturing its products for clinical and commercial usage, ensuring product availability and allowing Beacon to focus on clinical research.
Competition Analysis:
The Adeno Associated Virus (AAV) vector manufacturing market is characterized by a fragmented structure, with several players competing across various segments and regions. List of major players included in the Adeno Associated Virus (AAV) vector manufacturing market report are:
- Thermo Fisher Scientific Inc
- Cytiva
- Hoffman La Roche
- Charles River Laboratories
- Oxford Biomedica PLC
- WuXi AppTec
- Sarepta Therapeutics, Inc.
- AGC Biologics
- Genezen
- Lonza Group
- Catalent Biologics
- Creative Biogene
- Pfizer CentreOne
- Merck KGaA
Strategic Developments in Global Adeno Associated Virus (AAV) Vector Manufacturing Market:
- In October 2024, Ginkgo Bioworks, Inc., which is developing the leading platform for cell programming and biosecurity, and Virica Biotech Inc., a leading developer of enhancers for viral vector scaling as well as cell and gene therapies, have announced a strategic partnership to improve both companies’ adeno-associated virus (AAV) gene therapy manufacturing platforms. This partnership intends to address one of the primary challenges to widespread use of AAV-based gene therapies: high production costs.
- In May 2024, Siren Biotechnology, the pioneers of Universal AAV Immuno-Gene Therapy for Cancer, and Catalent Inc., the leader in enabling the development and supply of better treatments for patients worldwide, have formed a strategic alliance to support the development and manufacture of Siren Biotechnology’s AAV immuno-gene therapies. Catalent will provide process development and cGMP manufacturing services for Siren Biotechnology’s adeno-associated viral (AAV) vector-based treatment candidates in clinical trials. Catalent will provide additional support for process improvement at its process and clinical development hub in Baltimore, MD.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Global Adeno Associated Virus (AAV) Vector Manufacturing Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 1,148.4 Million |
| Market Growth Rate in CAGR (2025–2032) | 19.6% |
| Market Revenue forecast to 2032 | USD 4,829.1 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Global Adeno Associated Virus (AAV) Vector Manufacturing Market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Scale of Operations Outlook (Revenue, USD Million; 2022-2032)
- Clinical
- Preclinical
- Commercial
- Method Outlook (Revenue, USD Million; 2022-2032)
- Ex Vivo
- In Vivo
- Purification Outlook (Revenue, USD Million; 2022-2032)
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Therapeutic Area Outlook (Revenue, USD Million; 2022-2032)
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Application Outlook (Revenue, USD Million; 2022-2032)
- Gene Therapy
- Vaccine
- Cell Therapy
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Adeno Associated Virus (AAV) Vector Manufacturing market report
The market size of Adeno Associated Virus (AAV) vector manufacturing market was 1,148.4 million in 2024.
The market size of Adeno Associated Virus (AAV) vector manufacturing market is expected to register compound annual growth rate (CAGR) of 19.6% over the forecast period.
Increase in prevalence of chronic diseases worldwide, increasing demand for gene therapy, and technological advancements in AAV manufacturing are major key factors driving the market revenue growth of the global Adeno Associated Virus (AAV) vector manufacturing market.
High production costs and limited manufacturing capacity are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 22.0%.
Gene therapy is the major leading segment of Adeno Associated Virus (AAV) vector manufacturing market in terms of application.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Methods
- Primary
- Secondary
- Market Size Estimation
- Top-down method
- Bottom-up method
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Increase in prevalence of chronic disorders
- Increasing demand for gene therapy
- Technological advancements in AAV manufacturing
- Market Restraints
- High production costs
- Limited manufacturing capacity
- Market Opportunities
- Emergence of Contract Development and Manufacturing Organizations (CDMOs)
- Increasing focus on rare diseases
- Market Challenges
- Competition from alternative gene delivery technologies
- Potential immunogenicity risks
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Scale of Operations Market Revenue Estimates and Forecasts, 2022-2032
- Clinical
- Preclinical
- Commercial
- Method Market Revenue Estimates and Forecasts, 2022-2032
- Ex Vivo
- In Vivo
- Purification Market Revenue Estimates and Forecasts, 2022-2032
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Therapeutic Area Market Revenue Estimates and Forecasts, 2022-2032
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Application Market Revenue Estimates and Forecasts, 2022-2032
- Gene Therapy
- Vaccine
- Cell Therapy
- Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
- North America
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical
- Preclinical
- Commercial
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Method, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Ex Vivo
- In Vivo
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Purification, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Therapeutic Area, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Gene Therapy
- Vaccine
- Cell Therapy
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- North America Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- North America
- Europe
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical
- Preclinical
- Commercial
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Method, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Ex Vivo
- In Vivo
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Purification, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Therapeutic Area, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Gene Therapy
- Vaccine
- Cell Therapy
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia-Pacific
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical
- Preclinical
- Commercial
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Method, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Ex Vivo
- In Vivo
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Purification, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Therapeutic Area, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Gene Therapy
- Vaccine
- Cell Therapy
- Asia Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Asia-Pacific Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical
- Preclinical
- Commercial
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Method, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Ex Vivo
- In Vivo
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Purification, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Therapeutic Area, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Gene Therapy
- Vaccine
- Cell Therapy
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Latin America Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East & Africa
-
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical
- Preclinical
- Commercial
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Method, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Ex Vivo
- In Vivo
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Purification, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Chromatography
- Density Gradient Centrifugation
- Ion Exchange Chromatography
- Affinity Chromatography
- Ultrafiltration
- Chromatography
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Therapeutic Area, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Infectious Diseases
- Ophthalmic Disorders
- Hematological Diseases
- Others
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Application, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Gene Therapy
- Vaccine
- Cell Therapy
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Middle East & Africa Adeno Associated Virus (AAV) Vector Manufacturing Market By Scale of Operations, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- Thermo Fisher Scientific Inc
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Cytiva
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Hoffman La Roche
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Charles River Laboratories
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Oxford Biomedica PLC
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- WuXi AppTec
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sarepta Therapeutics, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- AGC Biologics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Genezen
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Lonza Group
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Catalent Biologics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Creative Biogene
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Pfizer CentreOne
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Merck KGaA
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

