Antisense Oligonucleotide (ASO) Therapeutics Market Overview and Key Insights:
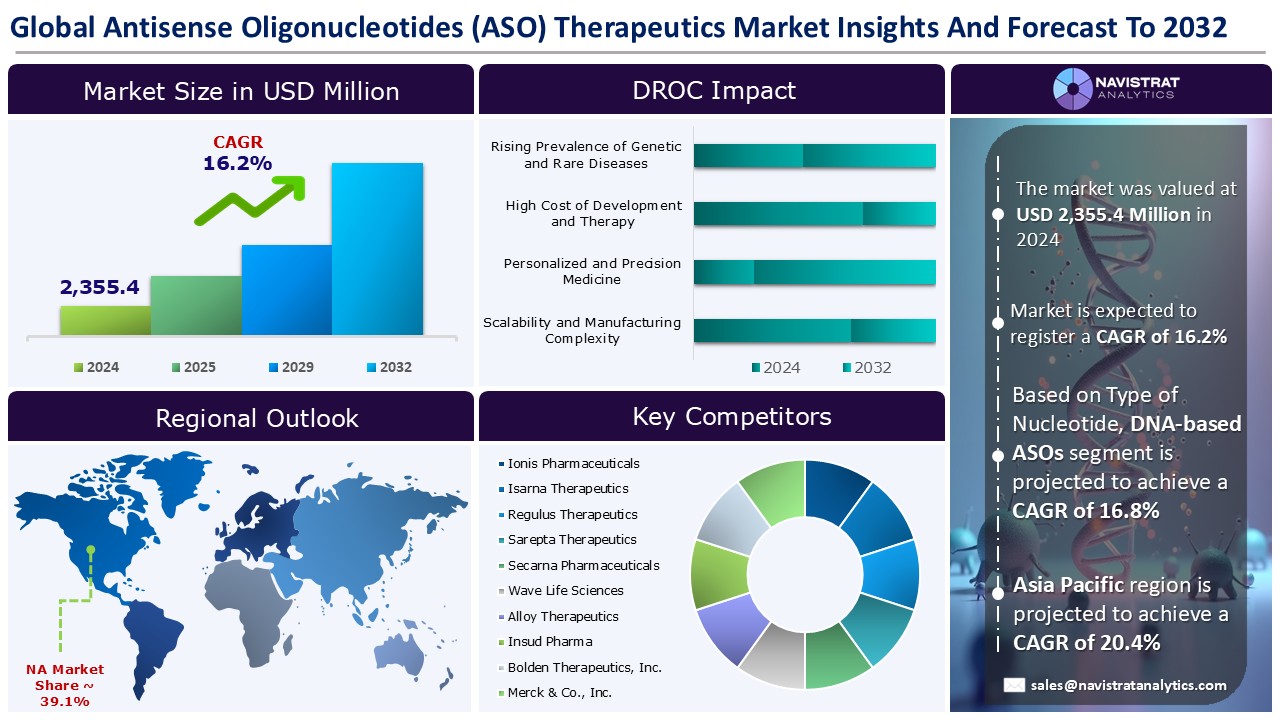
The Antisense Oligonucleotide (ASO) therapeutics market size reached USD 2,355.4 million in 2024 and is expected to register a revenue CAGR of 16.2% during the forecast period. Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides that can change RNA and reduce, restore, or regulate protein expression through various processes.
Market Drivers:
Antisense oligonucleotide developments provide a viable route for treating a wide range of genetic problems. As scientists and innovators delve further into the complexity of genetic illnesses, the importance of ASOs in providing focused, effective treatments becomes clearer. Recent advancements in systemic delivery systems, particularly enhanced exosome loading and nanoparticle technologies, open new therapeutic possibilities.
Rising prevalence of genetic and rare diseases is a key driver of revenue growth in the Antisense Oligonucleotide (ASO) therapeutics market. According to the American Lung Association, cystic fibrosis affects around 40,000 persons in the United States and an estimated 100,000 people worldwide. Approximately one in every thirty people in the United States is a carrier. Children born between 2019 and 2023 with cystic fibrosis are predicted to survive for an average of 61 years. Half of the newborns born in 2023 with cystic fibrosis are predicted to live to be 68 or older.
On February 2025, Biogen Inc. and Stoke Therapeutics, Inc. have announced a collaboration to develop and commercialize zorevunersen, a possible first-in-class disease-modifying drug in development for the treatment of Dravet syndrome, in all regions other than the United States, Canada, and Mexico. Zorevunersen is an experimental antisense oligonucleotide (ASO) that targets the SCN1A gene, which is the root cause of the majority of Dravet syndrome.
Market Opportunity:
Emerging delivery technologies acts as opportunities for Antisense Oligonucleotide (ASO) therapeutics market. Several lipid-based delivery technologies, including lipoplexes, liposomes, and lipid nanoparticles (LNP), have been widely employed to deliver ASOs and siRNAs. These NPs have shown remarkable properties, including the ability to cross multiple biological barriers and protect target genes from nuclease degradation, an improved pharmacokinetic profile by preventing renal excretion and RES clearance, increased stability in physiological solutions, and delivery to specific tissues or cells. Exosomes have recently emerged as a novel delivery mechanism for siRNA, ASOs, and small compounds to the brain.
Recent Trends:
Emerging trends include advancement in oligonucleotide chemistry, innovations in targeted delivery systems, CRISPR-ASO hybrid approaches, and splice modulation technology.
Splice-switching oligonucleotides (SSOs) are short, synthetic, antisense nucleic acids that base-pair with a pre-mRNA and disrupt the transcript’s normal splicing repertoire by interfering with RNA-RNA base-pairing or protein-RNA binding interactions between splicing machinery components and the pre-mRNA.
Splice-modulating ASOs have also been created for the treatment of inborn errors of metabolism (IEMs), owing to their capacity to redirect aberrant splicing induced by mutations, restoring normal transcript expression and rectifying functional protein deficiencies. Splice-modulating antisense oligonucleotides (ASOs) have presented a promising technique to create precision therapeutics for inborn metabolic abnormalities, such as lysosomal storage diseases, organic acidemias, and con-genital glycosylation disorders.
Restraints & Challenges:
ASOs have many potential applications as disease-modifying therapy for monogenic diseases. Limitations to the widespread use of ASOs include limited delivery options to affected tissues and a present lack of understanding of the required level of overexpression or downregulation of a specific protein to restore a disease phenotype. ASOs have low immunogenicity in general, although their interactions with the immune system can be influenced by their sequence, chemical changes, and mode of administration.
ASOs have been linked to a variety of toxicities, including hepatotoxicity, nephrotoxicity, thrombocytopenia, and local and systemic inflammation. Individual ASOs can induce distinct adverse reactions, but the class has several common harmful consequences.
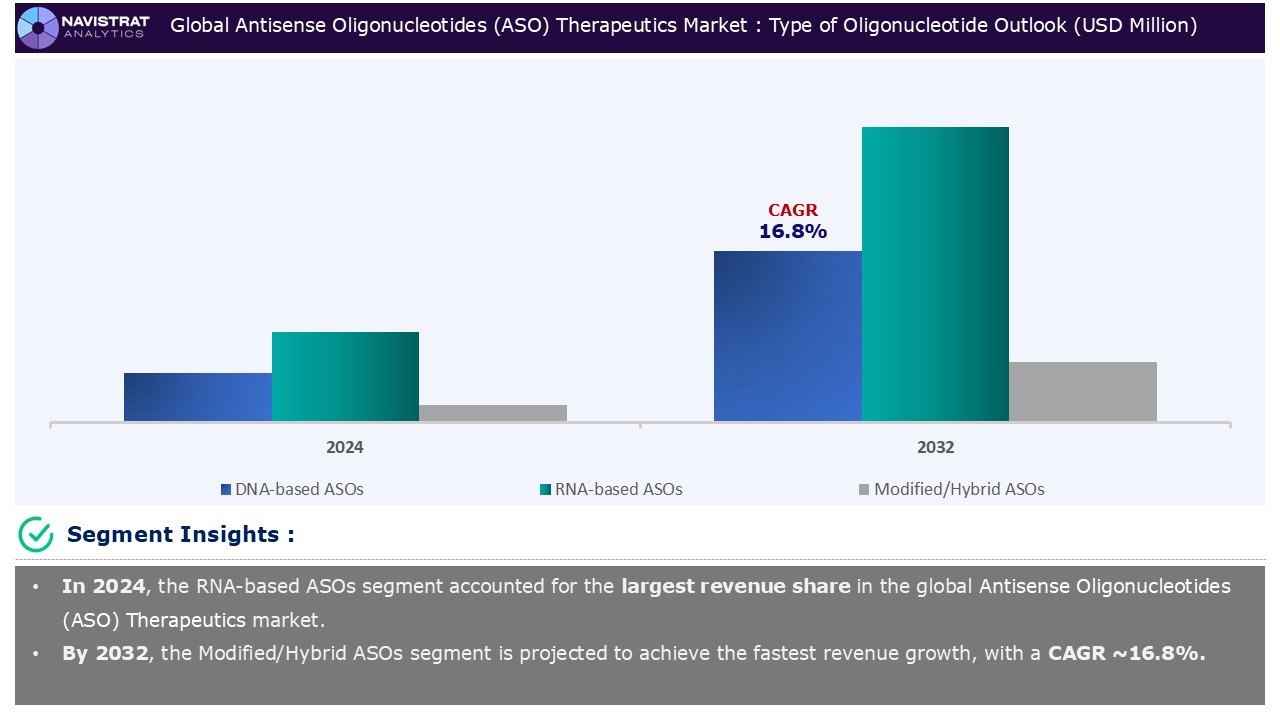
Type of Nucleotide Segment Insights and Analysis:
Based on the type of nucleotide, the Antisense Oligonucleotide (ASO) therapeutics market is segmented into DNA-based ASOs, RNA-based ASOs, and Modified/Hybrid ASOs.
RNA-based ASOs segment contributed the largest market share in 2024. RNA treatments, particularly antisense oligonucleotide (ASO) technology, have advanced dramatically in recent decades, with several ASOs being examined in various clinical stages and several ASO-based medications already on the market following FDA approval. The multiple activities of RNA, not only as a messenger of genetic information, but also as necessary and regulators of numerous stages of gene expression, are becoming clearer.
Synthesized ASOs can bind to complementary sequences in pre-mRNA, altering the recruitment of splicing factors to the molecule and regulating splicing events; bind to mature mRNA and prevent its attachment to the ribosome, blocking protein translation; or recruit RNase H to the target transcript, which will then be degraded.
On September 2024, HAYA Therapeutics, SA, a biotechnology company that pioneered precision RNA-guided regulatory genome targeting therapeutics for chronic diseases, has announced a multi-year agreement with Eli Lilly and Company to use HAYA’s advanced RNA-guided regulatory genome platform to support preclinical drug discovery efforts in obesity and related metabolic conditions. To treat these chronic illnesses, the partners will find various therapeutic targets based on regulatory genome-derived RNAs.
Technology Segment Insights and Analysis:
Based on the technology, the first-generation ASOs, second-generation ASOs, third-generation ASOs, and conjugated ASOs.
First-generation ASOs segment contributed the largest market share in 2024. First generation chemically modified ASOs include chemical groups such as methyl phosphonates (MP), phosphorothioate (PS), phosphoramidates, methylene (methylimino) (MMI), 5′N carbamate, triazole, amide, thioester, thioformacetal, mercapto acetamide, boranophosphate, 5′ methylurea, and guanidium. The alterations in the total charge of the phosphodiester backbone categorize the modifications as neutral, cationic, or anionic.
Phosphorothioate (PS) is the most common chemical modification of ASOs, arising from the substitution of a non-bridged oxygen atom with sulphur in the phosphodiester backbone. PS alteration confers various advantages, including increased nuclease resistance, improved solubility retention in water, and a longer half-life.
On June 2024, QurAlis Corporation has signed an exclusive license agreement with Eli Lilly and Company under which Lilly will have global rights to develop and commercialize QRL-204, a potentially best-in-class splice-switching antisense oligonucleotide (ASO) designed to restore UNC13A function in ALS, FTD, and other neurodegenerative diseases.
Indication Segment Insights and Analysis:
Based on the indication, the Antisense Oligonucleotide (ASO) therapeutics market is segmented into genetic disorders, neurological disorders, oncology, neurological disorders, infectious diseases, cardiovascular diseases, and others.
Neurological disorders segment contributed the largest market share in 2024. According to Alzheimer’s Association, alzheimer’s is the leading cause of dementia, accounting for approximately 60% to 80% of cases. About 5% to 10% of dementia patients have signs of vascular dementia alone. Approximately 60% of patients with FTD are between the ages of 45 and 60. According to a systematic review, FTD accounts for approximately 3% of dementia cases in research involving individuals aged 65 and older, and 10% in studies focusing on individuals under 65.
On April 2025, Biogen Inc. stated that the U.S. Food and Drug Administration (FDA) has given Fast Track designation to BIIB080, an experimental antisense oligonucleotide (ASO) therapy targeting tau for Alzheimer’s disease. The FDA granted Fast Track designation to accelerate the development and review of experimental medications that treat serious illnesses and address unmet medical needs. Biogen is developing BIIB080, the first tau-targeting ASO in clinical development for Alzheimer’s disease, and is currently testing it in the global Phase 2 CELIA study in people with early-stage illness.
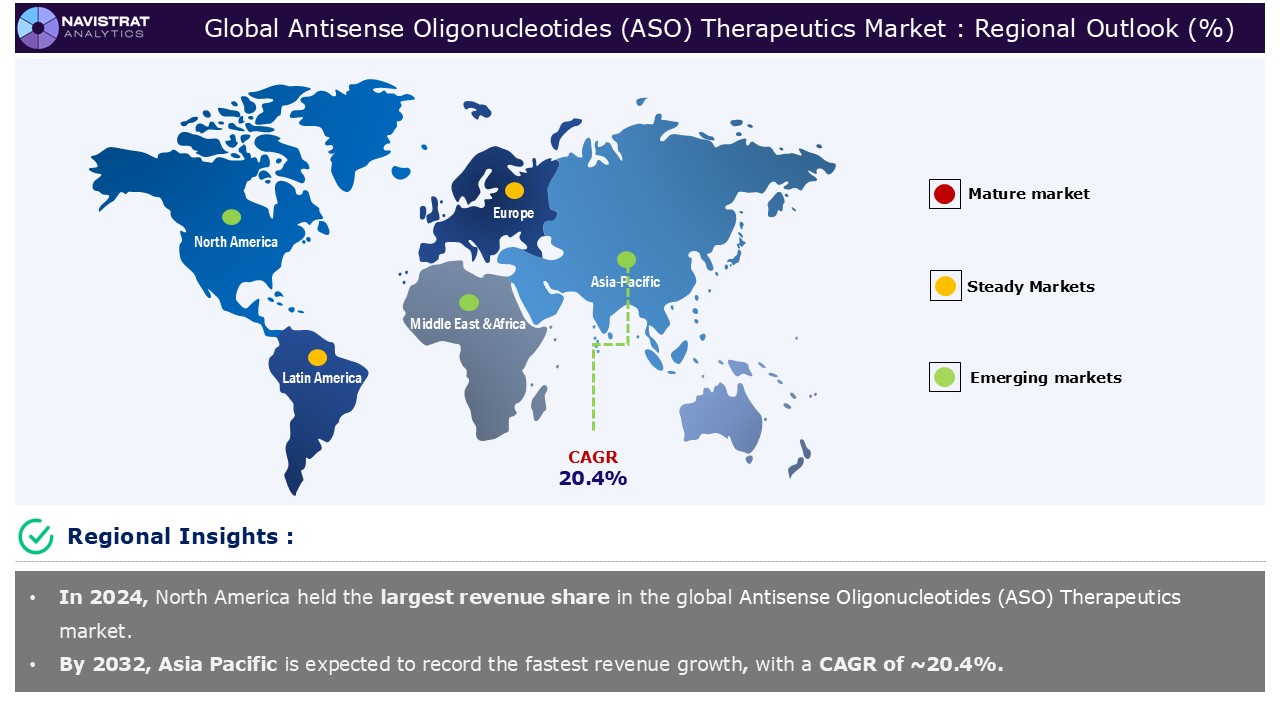
Geographical Outlook:
Antisense Oligonucleotide (ASO) therapeutics market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Antisense Oligonucleotide (ASO) Therapeutics Market:
North America is registered to have highest market share in Antisense Oligonucleotide (ASO) therapeutics market in 2024. The rising prevalence of genetic and rare diseases, along with advances in nucleic acid chemistry and delivery, mainly drive this trend. According to the National Center for Biotechnology Information (NCBI), Duchenne muscular dystrophy (DMD) follows an X-linked recessive inheritance pattern and more commonly affects boys than girls. The estimated incidence is one per 3600 male live-born newborns. Some studies suggest that DMD affects 2 out of every 10,000 people in the United States. It is one of the most prevalent and severe congenital myopathies.
On January 2024, Vanda Pharmaceuticals Inc. announced that the US Food and Drug Administration (FDA) has approved an Investigational New Drug (IND) application to test VCA-894A for the treatment of a patient with Charcot-Marie-Tooth disease, axonal, type 2S (CMT2S), which is caused by cryptic splice site variants in the IGHMBP2. VCA-894A is a new antisense oligonucleotide (ASO) that targets a cryptic splice site variation of immunoglobulin mu-binding protein 2 (IGHMBP2). Mutations in IGHMBP2 play a critical role in the development of CMT2S, which is most likely caused by alpha-motor neuron loss and, as a result, peripheral nervous system dysfunction.
Asia Pacific Antisense Oligonucleotide (ASO) Therapeutics Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period, driven by rising prevalence of genetic and rare diseases, and expanding pipeline of ASO candidates. According to the Joint United Nations Programme on HIV and AIDS, in 2023, there were 39.9 million [36.1 million-44.6 million] HIV-positive persons worldwide. In 2023, 1.3 million [1 million-1.7 million] new HIV infections occurred. 630 000 [500 000-820 000] individuals died from AIDS-related illnesses in 2023. The global median HIV prevalence among adults aged 15 to 49 was 0.8%. However, due to marginalization, discrimination, and, in certain cases, criminality, certain groups had a greater median frequency.
On April 2025, Hoth Therapeutics, Inc., a clinical-stage biopharmaceutical company developing next-generation RNA-targeted precision treatments, announced that the Japan Patent Office (JPO) has granted it a crucial patent, strengthening its global intellectual property portfolio in RNA-based cancer therapeutics. The newly awarded patent includes extensive claims for antisense oligomers that target the KIT gene, a clinically confirmed oncogene linked to gastrointestinal stromal tumors (GIST), leukemia, mastocytosis, and other malignancies. These oligomers are intended to change pre-mRNA splicing or limit KIT protein production, providing a highly specific treatment approach.
Europe Antisense Oligonucleotide (ASO) Therapeutics Market:
Europe is expected to have considerable market share in 2024. Leading companies in Europe are prioritizing strategic mergers, acquisitions, and partnerships to strengthen their antisense oligonucleotide portfolios and expand their product capabilities. On March 2024, Celanese Corporation, a global specialty materials and chemical company, and Secarna Pharmaceuticals GmbH & Co. KG, a leading independent European antisense drug discovery and development company, have announced a research collaboration to develop long-acting implants containing antisense oligonucleotides (ASOs). Celanese will use its VitalDose Drug Delivery Platform together with Secarna’s proprietary ASO Drug Discovery and Development Platform to create ASO-eluting implants. These implants aim to reduce dosing frequency, minimize off-target immune responses, and enhance targeting, ultimately improving patient outcomes across various indications.
Competition Analysis:
The Antisense Oligonucleotide (ASO) therapeutics market is characterized by a fragmented structure, with several players competing across various segments and regions. list of major players included in the Antisense Oligonucleotide (ASO) therapeutics market report are:
- Ionis Pharmaceuticals
- Isarna Therapeutics
- Regulus Therapeutics
- Sarepta Therapeutics
- Secarna Pharmaceuticals
- Wave Life Sciences
- Alloy Therapeutics
- Insud Pharma
- Bolden Therapeutics, Inc.
- Merck & Co., Inc.
- Biogen Inc.
- Arrowhead Pharmaceuticals
- Hoffmann-La Roche
- Nippon Shinyaku
- Alnylam Pharmaceuticals
Strategic Developments in Antisense Oligonucleotide (ASO) Therapeutics Market:
- In November 2024, Trace Neuroscience, Inc., a biopharmaceutical company that is expanding the promise of genomic medicine for people living with neurodegenerative diseases, has announced the launch of a USD 101 million Series A financing led by Third Rock Ventures, with participation from Atlas Venture, GV, and RA Capital Management. Trace Neuroscience’s flagship program is an antisense oligonucleotide (ASO) aimed to preserve and potentially improve muscular function in persons living with amyotrophic lateral sclerosis (ALS), particularly those with the sporadic form, which affects nine out of ten people with the condition.
- In June 2024, QurAlis Corporation, a clinical-stage biotechnology company that is translating scientific breakthroughs into powerful precision medicines with the potential to change the course of amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and other neurodegenerative and neurological diseases, has announced that it is expanding its industry-leading precision medicine antisense oligonucleotide (ASO) splicing expertise beyond neurodegeneration to Fragile X syndrome (FXS).
- In January 2024, Bolden pharmaceuticals, Inc., a biotechnology firm researching first-in-class pharmaceuticals to stimulate neurogenesis for the potential treatment of central nervous system diseases such as Alzheimer’s disease, has announced the completion of a USD 1.5 million pre-seed convertible note financing. This funding will allow Bolden to continue its preclinical study of antisense oligonucleotides to stimulate neurogenesis. Resolute Venture Partners led the round, which includes investments from Slater Technology Fund, Lifespan Vision Ventures, and many angel investors.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Antisense Oligonucleotide (ASO) Therapeutics Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 2,355.4 Million |
| Market Growth Rate in CAGR (2025–2032) | 16.2% |
| Market Revenue forecast to 2032 | USD 7,864.9 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Antisense Oligonucleotide (ASO) Therapeutics market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Type of Nucleotide Outlook (Revenue, USD Million; 2022-2032)
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Technology Outlook (Revenue, USD Million; 2022-2032)
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Route of Administration Outlook (Revenue, USD Million; 2022-2032)
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Development Phase Outlook (Revenue, USD Million; 2022-2032)
- Preclinical
- Clinical
- Commercial
- Indication Outlook (Revenue, USD Million; 2022-2032)
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Antisense Oligonucleotide (ASO) Therapeutics market report
The market size of Antisense Oligonucleotide (ASO) therapeutics market was 2,355.4 million in 2024.
The market size of Antisense Oligonucleotide (ASO) Therapeutics market is expected to register compound annual growth rate (CAGR) of 16.2% over the forecast period.
Rising prevalence of genetic and rare diseases, advances in nucleic acid chemistry and delivery, and increased regulatory support and orphan drug designations are major key factors driving the market revenue growth of the Antisense Oligonucleotide (ASO) therapeutics market.
High cost of development and therapy and stringent regulatory and safety hurdles are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 20.4%.
RNA-based ASOs is the major leading segment of Antisense Oligonucleotide (ASO) therapeutics market in terms of type of nucleotide.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Methods
- Primary
- Secondary
- Market Size Estimation
- Top-down Technology
- Bottom-up Technology
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Rising demand for nucleic acid-based therapies
- Advancements in manufacturing technologies
- Increasing outsourcing to CDMOs & CMOs
- Market Restraints
- High manufacturing costs
- Limited skilled workforce and infrastructure
- Market Opportunities
- Expansion of mRNA and gene editing therapies
- Rising demand for non-viral delivery technologies
- Government funding and investments
- Market Challenges
- Scalability and production bottlenecks
- Raw material supply chain issues
- Competition from in-house manufacturing
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Type of Oligonucleotide Market Revenue Estimates and Forecasts, 2022-2032
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Technology Market Revenue Estimates and Forecasts, 2022-2032
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Route of Administration Market Revenue Estimates and Forecasts, 2022-2032
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Development Phase Market Revenue Estimates and Forecasts, 2022-2032
- Preclinical
- Clinical
- Commercial
- Indication Market Revenue Estimates and Forecasts, 2022-2032
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
-
- North America
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Route of Administration, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Development Phase, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Preclinical
- Clinical
- Commercial
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- North America Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- North America Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- North America
- Europe
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Route of Administration, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Development Phase, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Preclinical
- Clinical
- Commercial
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia-Pacific
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Route of Administration, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Development Phase, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Preclinical
- Clinical
- Commercial
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Asia-Pacific Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Route of Administration, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Development Phase, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Preclinical
- Clinical
- Commercial
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Latin America Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East & Africa
-
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- DNA-based ASOs
- RNA-based ASOs
- Modified/Hybrid ASOs
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Technology, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- First-generation ASOs
- Second-generation ASOs
- Third-generation ASOs
- Conjugated ASOs
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Route of Administration, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Intrathecal
- Intravenous (IV)
- Subcutaneous
- Intraocular
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Development Phase, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Preclinical
- Clinical
- Commercial
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Genetic Disorders
- Neurological Disorders
- Oncology
- Neurological Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Middle East & Africa Antisense Oligonucleotide (ASO) Therapeutics Market By Type of Oligonucleotide, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- Ionis Pharmaceuticals
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Isarna Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Regulus Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sarepta Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Secarna Pharmaceuticals
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Wave Life Sciences
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Alloy Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Insud Pharma
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Bolden Therapeutics, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Merck & Co., Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Biogen Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Arrowhead Pharmaceuticals
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Hoffmann-La Roche
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Nippon Shinyaku
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Alnylam Pharmaceuticals
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

