Oligonucleotide CDMO Market Overview and Key Insights:
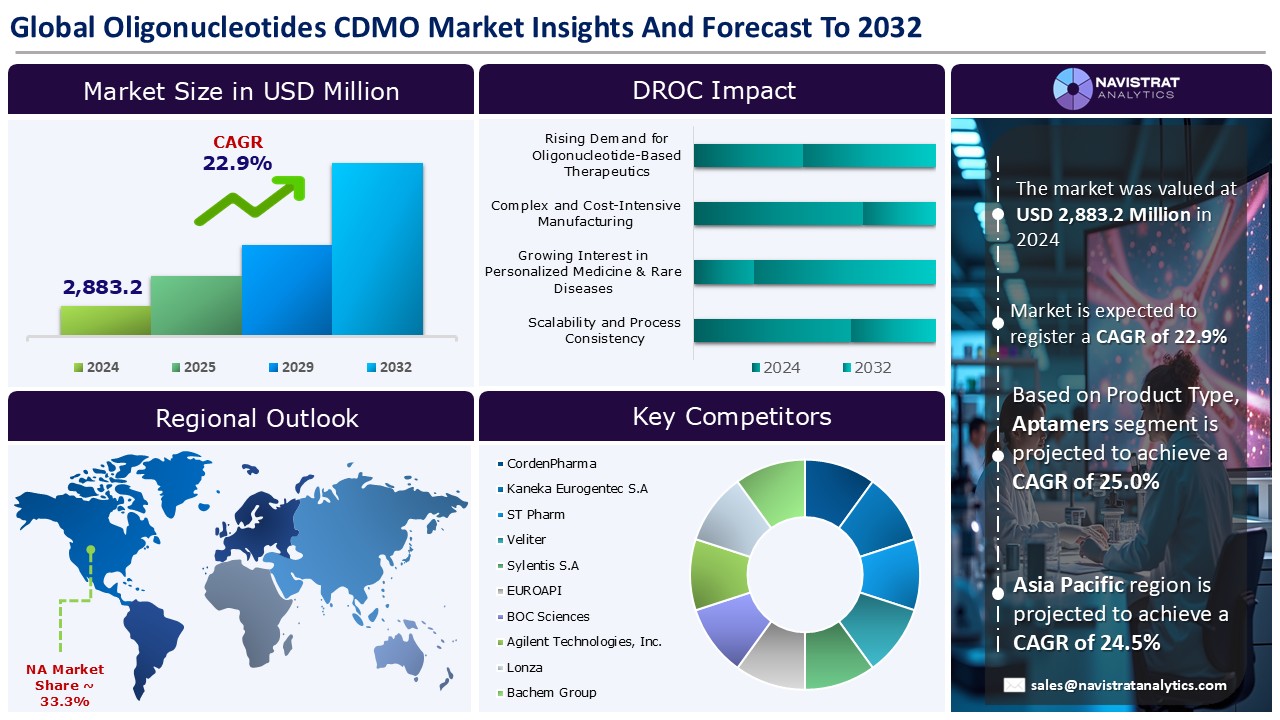
The oligonucleotide CDMO market size reached USD 2,883.2 million in 2024 and is expected to register a revenue CAGR of 22.9% during the forecast period. Oligonucleotide (oligo) therapies are synthesized DNA or RNA strands that bind to a specific gene or protein sequence. Oligonucleotides, which comprise DNA-based ASOs, small interfering RNAs (siRNAs), and other oligonucleotide variations, have excellent specificity and show promise for treating malignancies, genetic illnesses, and other conditions.
Market Drivers:
Increase in prevalence of chronic disorders is a key driver of revenue growth in the oligonucleotide CDMO market. According to the Spinal Muscular Atrophy (SMA) Association, has been estimated to impact between 10,000 and 25,000 children and adults in the United States, making it one of the most frequent uncommon disorders. The condition affects one out of every 6,000 to ten thousand children born. The SMA gene is carried by one in every 40 to 50 persons (about 6 million Americans). Furthermore, Huntington Disease (HD) prevalence is estimated at 6.37 per 100,000 people in Europe and 8.87 per 100,000 people in North America. In contrast, it is estimated at 0.25 per 100,000 people in Africa, 0.41 per 100,000 people in East Asia, and 2.39 per 100,000 people when the Middle East and East Asia are combined.
Oligonucleotide therapies are now widely used across pipelines, entity kinds, and clinical trials. Cloud and digital twin-based resource management of materials, products, operations, staff, and production schedules improves green manufacturing by optimizing process efficiency, energy use timing, net energy demand, and product batches per facility or time. The recent success of AI and machine learning in pharmaceutical operations, particularly the empowering of digital twins, provides even larger prospects for both design and operational efficiency.
On May 2023, GenScript Biotech Corporation, the world’s largest provider of life science research equipment and services, has expanded its principal manufacturing facility for oligonucleotide and peptide manufacture in Zhenjiang, Jiangsu, China. The expansion builds on GenScript’s 20-year record for providing high-quality oligo and peptides to scientists around the world. The increased oligonucleotide synthesis capabilities provide qPCR oligos, NGS oligos, RNA oligos, DNA oligos, and other options to support a wide range of applications, including molecular diagnostics, RNAi, and genome editing.
Market Opportunity:
Emergence of novel delivery systems acts as opportunities for oligonucleotide CDMO market. The optimization of oligonucleotides and their delivery mechanisms is critical for increasing their therapeutic efficacy in genetic disorders, neurological illnesses, and cancer. Advances in oligonucleotide therapy have resulted in improved chemical modifications, target specificity, and drug delivery techniques, ensuring precise gene silencing with minimal off-target consequences.
Researchers have developed several methods to improve oligonucleotide distribution. They add nucleic acid medicines to the aqueous compartment of liposomes. Many have attempted to use liposomes for oligonucleotide delivery because liposomes effectively protect oligonucleotides from nuclease-mediated degradation and enhance cellular absorption efficiency.
Recent Trends:
Emerging trends include integration of AI and automation, next generation delivery methods, and technological advancements in oligonucleotides manufacturing.
Artificial intelligence-driven research, optimization, and manufacturing breakthroughs have greatly sped Antisense oligonucleotide (ASO) discovery, increased treatment efficacy, improved delivery systems, and lowered production costs. The use of advanced AI models, such as OpenAI o1/o3, Gemini 2.0, Llama 3.2/3.3, reinforcement learning (RL), graph neural networks (GNNs), diffusion models, and multi-agent AI systems, has transformed ASO development, clinical translation, and regulatory compliance.
On September 2024, Atinary Technologies, whose premier no-code AI platform, SDLabs, and Self-Driving Labs technologies are redefining new molecule and chemical R&D, has announced a strategic partnership with Snapdragon Chemistry, a Cambrex firm. Snapdragon combined its proprietary LabOS software platform with Atinary’s SDLabs platform to create a fully automated and AI-driven, medium-throughput Solid-Phase Oligonucleotide Synthesizer (SPOS) for autonomous multi-objective process optimization.
Restraints & Challenges:
Complex and cost-intensive manufacturing is one of the major concerns in the field of oligonucleotide therapeutics. Despite increased interest in oligonucleotide-based therapies, worldwide production capacity remains restricted. The high cost of raw materials necessary for oligonucleotide synthesis is one of the key causes of high production costs. High-purity reagents and specific solvents are required to ensure nucleotide incorporation integrity during synthesis. Longer and more complicated oligos necessitate more expensive reagents.
Some fundamental characteristics of oligonucleotide manufacture are difficult to overcome using green chemistry solutions, such as the vast volumes of organic and aqueous waste produced during synthesis and purification. Furthermore, the intricacy of oligonucleotide structures precludes the use of new green chemistry techniques like enzymatic synthesis, which could provide more sustainable solutions.
Product Type Segment Insights and Analysis:
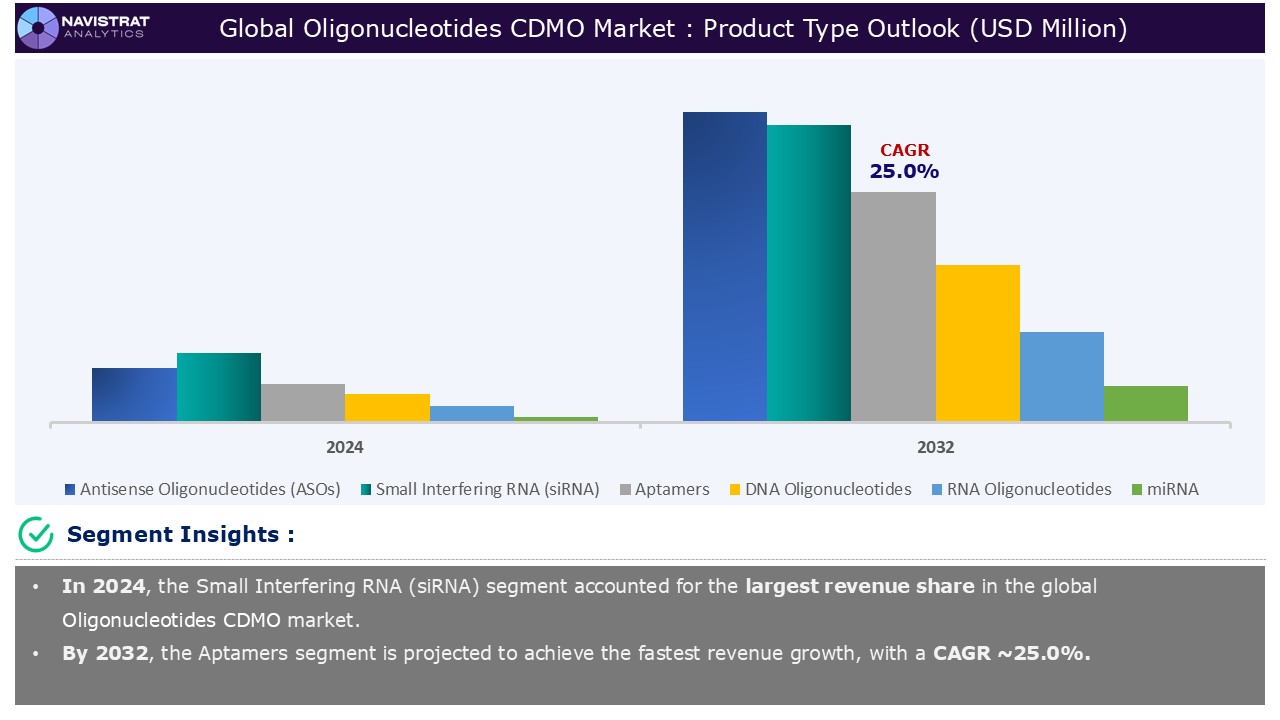
Based on the product type, the Oligonucleotide CDMO market is segmented into Antisense Oligonucleotides (ASOs), Small Interfering RNA (siRNA), Aptamers, DNA Oligonucleotides, RNA Oligonucleotides, and miRNA.
Small Interfering RNA (siRNA) segment contributed the largest market share in 2024. siRNA-based therapies for viral infections are particularly promising and flexible. siRNA therapeutics provide adaptable approaches to viral infection treatment. The RNA-induced silencing complex (RISC) mediates the cleavage of virally encoded cytoplasmic mRNAs, which inhibits virus replication. Exogenous siRNAs destroy viral RNAs using RNAi mechanisms. As soon as the genome of a new virus is discovered, RNAi becomes a reliable infection-prevention method. In both preventive and curative situations, siRNA-based RNAi therapeutics target the source of infection rather than just treating the symptoms.
On January 2024, Pluri Inc., a leading biotech company that transforms cells into solutions that promote global well-being and sustainability, announced the launch of PluriCDMO, a new business division that provides cell therapy manufacturing services as a Contract Development and Manufacturing Organization (CDMO). PluriCDMO will provide its unique knowledge and technology, as well as more than 20 years of development and manufacturing experience. The company operates a cutting-edge 47,000-square-foot Good Manufacturing Practice (GMP) cell therapy production facility to help clients and partners overcome critical challenges in developing and manufacturing cell-based medicines.
Service Type Segment Insights and Analysis:
Based on the service type, the oligonucleotide CDMO market is segmented into custom oligonucleotide synthesis, process development & optimization, analytical services, manufacturing services, and others.
Custom oligonucleotide synthesis segment contributed the largest market share in 2024. Researchers, pharmaceutical companies, and clinical trial teams customize oligonucleotides through a process called custom oligonucleotide synthesis. This process allows them to create specific RNA or DNA sequences for use in gene therapy, diagnostics, and various other applications.
The capacity to create custom oligos has transformed how scientists approach genetic research and medicine development. Companies can use designed oligonucleotides to target specific genes, investigate genetic mutations, and build personalized medicines for individuals with genetic illnesses. For example, in gene therapy, researchers frequently use custom oligonucleotide synthesis to generate molecules that can modify or repair faulty genes, offering hope for previously untreatable diseases.
Indication Segment Insights and Analysis:
Based on the indication, the oligonucleotide CDMO market is segmented into oncology, rare & genetic disorders, infectious diseases, neurological disorders, cardiovascular diseases, and ophthalmic diseases.
Neurological disorders segment contributed the largest market share in 2024. Duchenne muscular dystrophy (DMD) is a rare muscle ailment, yet it is one of the most common hereditary conditions, affecting roughly one in every 3,500 male births globally. In Europe and North America, the prevalence of DMD is around 6 per 100,000. Duchenne affects roughly 1 in every 5,000 live male births.
ASO therapy has made amazing advances in recent years, providing major advantages in the treatment of motor neuron disorders. The greatest success has been the invention of nusinersen, the first effective medication for SMA approved by the FDA and EMA, which can relieve symptoms and decrease disease progression.
On August 2023, EUROAPI and the shareholders of BianoGMP, a Contract Development and Manufacturing Organization with recognized expertise in oligonucleotides, have inked a share acquisition and transfer agreement under which EUROAPI will acquire all Biano shares. This acquisition would boost EUROAPI’s CDMO market position in oligonucleotides by including a recognized oligonucleotide CDMO pure player with growth potential.
Geographical Outlook:
Oligonucleotide CDMO market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Oligonucleotide CDMO Market:
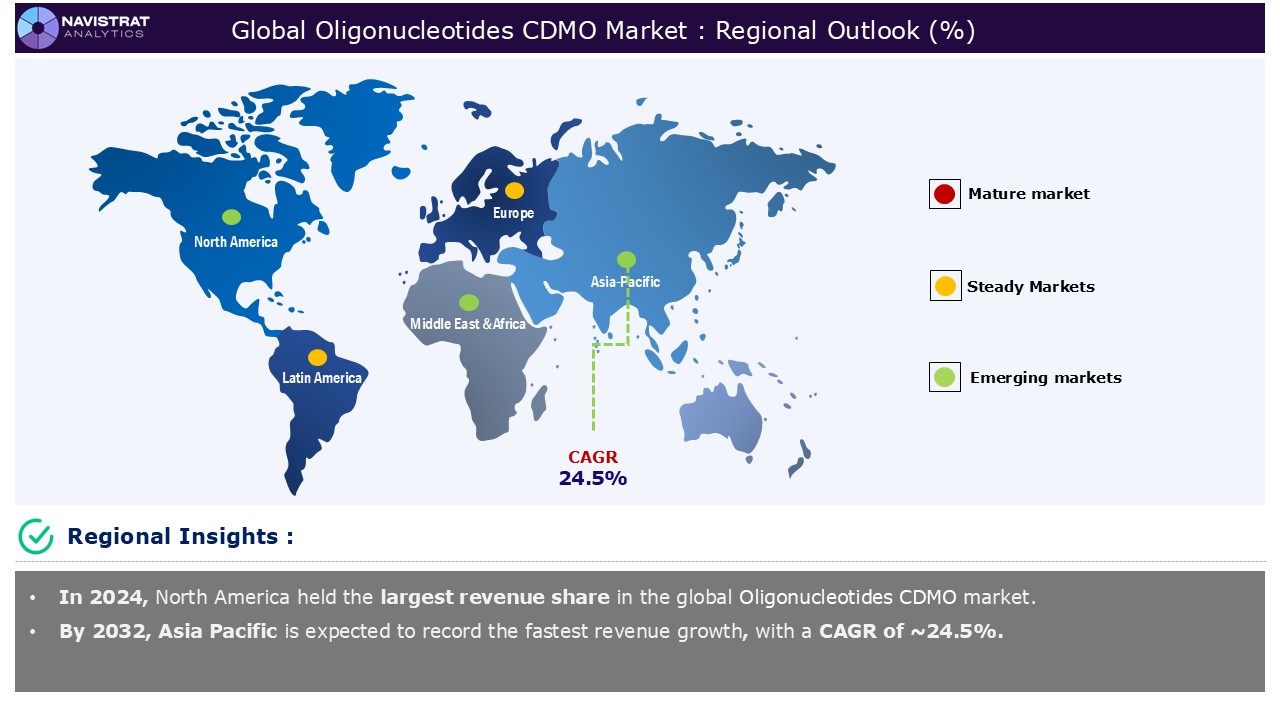
North America is registered to have the largest market share in oligonucleotide CDMO market in 2024. This is mainly driven by rising demand for oligonucleotide-based therapeutics and technological advancements in oligonucleotide synthesis. According to the National Organization for Rare Disorder (NORD), a rare disease is a problem, illness, or condition that affects less than 200,000 Americans. There are about 10,000 uncommon diseases affecting more than 30 million Americans.
On September 2024, Eurofins Genomics US has announced the opening of a new world-class oligonucleotide manufacturing plant. The expansion greatly expands Eurofins Genomics US’s manufacturing capacity and capabilities, allowing the company to fulfill the world’s expanding demand for GMP-grade and research-use oligonucleotides. The company designed the new GMP facility to provide a cutting-edge environment for manufacturing synthetic DNA and RNA, ensuring the highest levels of quality, safety, and efficiency. The new facility’s architecture incorporates totally separate production trains for Research Use Only (RUO) and Good Manufacturing Practice (GMP) items.
Asia Pacific Oligonucleotide CDMO Market:
Asia Pacific is expected to register the fastest growth rate during the forecasted period, driven by increased outsourcing by biopharma companies and rising demand for oligonucleotide-based therapeutics. The biopharmaceutical industry is shifting towards novel drug modalities, which are projected to outperform traditional small-molecule medications in the future decade. India and the greater APAC region are likely to have tremendous growth in a variety of healthcare industries.
On January 2023, Aurisco, a China-based innovative pharmaceutical firm and CDMO, has announced a collaboration with Cytiva, a global science leader, to develop its first Oligo FlexFactory platform for commercial production. With increased speed and efficiency, Aurisco will be able to deliver more oligonucleotides and better serve its customers across the world. Aurisco has established the Oligo FlexFactory platform as the first of three planned commercial manufacturing lines in Yangzhou, East China. The company plans to produce 200 kilos of oligonucleotides annually at the entire production facility.
Europe Oligonucleotide CDMO Market:
Europe is expected to have considerable market share in 2024, driven by the growing need for biologics and personalized medicine, along with economic concerns, is driving pharmaceutical companies to increasingly outsource to CDMOs. European countries such as Germany and Switzerland, has established itself as a leader in CDMO services. Companies like BioSpring, Lonza, Bachem, and Eurofins Genomics offer scalable production options.
On February 2025, Recipharm, a leading global contract development and manufacturing organization (CDMO), has announced the creation of a new Pilot Scale Development Center in Germany. Following the announcement of the investment last year, the new equipment is now fully operational, expanding Recipharm’s product development (PD) capabilities and reaffirming the company’s commitment to providing high-quality pharmaceutical solutions. The new Pilot Scale Development Centre expands Recipharm’s existing product development capabilities, assuring customers of a smooth transition from PD to commercial manufacture.
Competition Analysis:
The oligonucleotide CDMO market is characterized by a fragmented structure, with several players competing across various segments and regions. list of major players included in the oligonucleotide CDMO market report are:
- CordenPharma
- Kaneka Eurogentec S.A
- ST Pharm
- Veliter
- Sylentis S.A
- EUROAPI
- BOC Sciences
- Agilent Technologies, Inc.
- Lonza
- Bachem Group
- NIPPON SHOKUBAI CO., LTD.
- TAPI
- Syngene International Limited
- CPC Scientific Inc.
- Aurisco
Strategic Developments in Oligonucleotide CDMO Market:
- In February 2025, Sumitomo Chemical launched a new company, Sumitomo Chemical Advanced Medical Solutions America LLC, in Marlborough, Massachusetts. This company will act as a CRO for Sumitomo Chemical’s oligonucleotide CDMO business. The Company has invented the world’s first technique to produce at scale long-chain gRNAs that are several times longer (about 100 mer*3) than typical nucleic acid therapeutic ingredients, with high purity of around 90% and high yield.
- In November 2024, Lonza, a global development and manufacturing partner for the pharmaceutical, biotech, and nutraceutical industries, reported that it will invest in further bioconjugation capabilities in Visp (CH). The extension will include two multipurpose 1,200L manufacturing suites as well as manufacturing-related infrastructure for the current bioconjugation plant in Visp (CH), allowing for launch and commercial supply. The increased capacity will create roughly 200 new jobs and is projected to be operational by 2028.
- In September 2024, Agilent Technologies Inc. announced the completion of its acquisition of BIOVECTRA, a contract development and manufacturing organization (CDMO) based in Canada that focuses on biologics, highly potent active pharmaceutical ingredients, and other compounds for targeted treatments. With the integration of BIOVECTRA, Agilent will broaden its CDMO service offering, add rapidly developing modalities, and provide world-class capabilities for gene editing.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights Of The Oligonucleotide CDMO Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 2,883.2 Million |
| Market Growth Rate in CAGR (2025–2032) | 22.9% |
| Market Revenue forecast to 2032 | USD 15,071.7 Million |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
|
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Oligonucleotide CDMO market report offers a detailed analysis of market size, including historical revenue (in USD Million) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Product Type Outlook (Revenue, USD Million; 2022-2032)
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Service Type Outlook (Revenue, USD Million; 2022-2032)
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Synthesis Scale Outlook (Revenue, USD Million; 2022-2032)
- Clinical Stage
- Commercial Stage
- Indication Outlook (Revenue, USD Million; 2022-2032)
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Regional Outlook (Revenue, USD Million; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Oligonucleotide CDMO market report
The market size of oligonucleotide CDMO market was 2,883.2 million in 2024.
The market size of oligonucleotide CDMO market is expected to register compound annual growth rate (CAGR) of 22.9% over the forecast period.
Rising demand for oligonucleotide-based therapeutics, increased outsourcing by biopharma companies, and technological advancements in oligonucleotide synthesis are major key factors driving the market revenue growth of the oligonucleotide CDMO market.
Complex and cost-intensive manufacturing and limited global manufacturing capacity are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 24.5%.
Small Interfering RNA (siRNA) is the major leading segment of oligonucleotide CDMO market in terms of product type.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Methods
- Primary
- Secondary
- Market Size Estimation
- Top-down Synthesis Scale
- Bottom-up Synthesis Scale
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Rising demand for oligonucleotide-based therapeutics
- Increased outsourcing by biopharma companies
- Technological advancements in oligonucleotide synthesis
- Market Restraints
- Complex and cost-intensive manufacturing
- Limited global manufacturing capacity
- Market Opportunities
- Growing interest in personalized medicine & rare diseases
- Emergence of novel delivery systems
- Market Challenges
- Scalability and Process Consistency
- Waste Management and Environmental Concerns
- Shortage of Skilled Workforce
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Product Type Market Revenue Estimates and Forecasts, 2022-2032
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Service Type Market Revenue Estimates and Forecasts, 2022-2032
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Synthesis Scale Market Revenue Estimates and Forecasts, 2022-2032
- Clinical Stage
- Commercial Stage
- Indication Market Revenue Estimates and Forecasts, 2022-2032
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Million
- North America
- North America Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- North America Oligonucleotide CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- North America Oligonucleotide CDMO Market By Synthesis Scale, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical Stage
- Commercial Stage
- North America Oligonucleotide CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- North America Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- United States
- Canada
- Mexico
- North America Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Europe
- Europe Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Europe Oligonucleotide CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Europe Oligonucleotide CDMO Market By Synthesis Scale, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical Stage
- Commercial Stage
- Europe Oligonucleotide CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Europe Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Asia-Pacific
- Asia-Pacific Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Asia-Pacific Oligonucleotide CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Asia-Pacific Oligonucleotide CDMO Market By Synthesis Scale, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical Stage
- Commercial Stage
- Asia-Pacific Oligonucleotide CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Asia-Pacific Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Asia-Pacific Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Latin America
- Latin America Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Latin America Oligonucleotide CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Latin America Oligonucleotide CDMO Market By Synthesis Scale, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical Stage
- Commercial Stage
- Latin America Oligonucleotide CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Latin America Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- Brazil
- Rest of Latin America
- Latin America Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Middle East & Africa
- North America
-
-
- Middle East & Africa Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Antisense Oligonucleotides (ASOs)
- Small Interfering RNA (siRNA)
- Aptamers
- DNA Oligonucleotides
- RNA Oligonucleotides
- miRNA
- Middle East & Africa Oligonucleotide CDMO Market By Service Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Custom Oligonucleotide Synthesis
- Process Development & Optimization
- Analytical Services
- Manufacturing Services
- Others
- Middle East & Africa Oligonucleotide CDMO Market By Synthesis Scale, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Clinical Stage
- Commercial Stage
- Middle East & Africa Oligonucleotide CDMO Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
- Oncology
- Rare & Genetic Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Diseases
- Ophthalmic Diseases
- Middle East & Africa Oligonucleotide CDMO Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Million
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Middle East & Africa Oligonucleotide CDMO Market By Product Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Million
-
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- CordenPharma
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Kaneka Eurogentec S.A
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- ST Pharm
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Veliter
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sylentis S.A
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- EUROAPI
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- BOC Sciences
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Agilent Technologies, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Lonza
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Bachem Group
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- NIPPON SHOKUBAI CO., LTD.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- TAPI
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Syngene International Limited
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- CPC Scientific Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Aurisco
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

