Cancer Immunotherapy Market Overview and Key Insights:
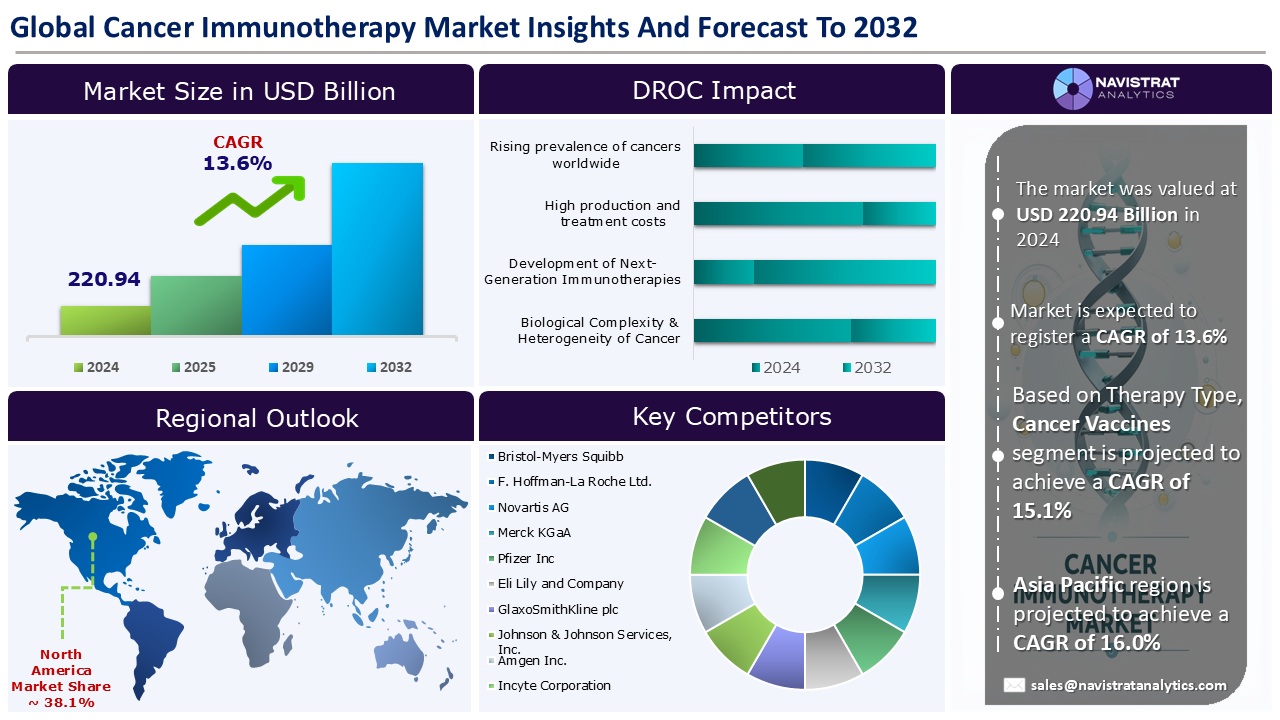
The cancer immunotherapy market size reached USD 220.94 billion in 2024 and is expected to register a revenue CAGR of 13.6% during the forecast period. Immunotherapy is a novel treatment that dynamically regulates the immune system to attack cancer cells in many targets and orientations. Immunotherapy is primarily utilized to enhance the immune system by modulating the immunological microenvironment, allowing immune cells to attack and eliminate tumor cells at numerous key nodes.
Market Drivers:
Rising prevalence of cancers worldwide is a key driver of revenue growth in the cancer immunotherapy market. According to the American Cancer Society, in 2025, the US is anticipated to have over 2 million new cancer diagnoses and 618,000 fatalities, excluding non-melanoma skin cancers. This equates to over 1,700 deaths each day. In 2025, the US is expected to have 316,950 new cases of invasive breast cancer in women and 2,800 in men, as well as 59,080 cases of ductal carcinoma in situ (DCIS) in women. In 2025, a projected 42,680 people will die from breast cancer, with 42,170 women and 510 males.
The future of cancer immunotherapy seems optimistic, with a focus on tailored and combination methods to improve treatment efficacy and address present hurdles. Personalized immunotherapies, such as neoantigen-targeting and patient-specific cancer vaccines, hold the promise of highly individualized therapy regimens. The combination of CAR-T cell treatment and immune checkpoint inhibitors (ICIs) provides a potential to improve CAR-T cell persistence and function, particularly in solid tumors.
Emerging fields of research include leveraging the gut microbiome to improve immunotherapy and using oncolytic viruses to selectively target cancer cells. Furthermore, broadening cellular immunotherapy to include other immune cells such as natural killer (NK) cells opens the possibility of several methods of action against cancers.
On January 2025, Astellas Pharma Inc. announced that the National Medical Products Administration (NMPA) of China has approved VYLOY (zolbetuximab) in combination with fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma with claudin (CLDN) 18.2 positive tumors. Zolbetuximab is the first NMPA-approved monoclonal antibody to target gastric tumor cells expressing the biomarker CLDN18.2, providing a highly tailored approach to cancer treatment.
Market Opportunity:
Integration of Artificial Intelligence (AI) & big data in drug discoveries acts as an opportunity for the cancer immunotherapy market. AI is increasingly being used in cancer immunotherapy, providing unprecedented opportunity to improve treatment efficacy, forecast patient responses, and identify new therapeutic targets. Traditionally, biomarker development has been a time-consuming procedure, hampered by the complexities of biological data and the requirement for advanced analytical techniques. AI, particularly through ML and deep-learning (DL) algorithms, has changed this process by allowing the quick analysis of large-scale genomic, transcriptomic, and proteomic data to find novel biomarkers that could be overlooked using conventional methods.
On December 2024, HealthCare Global Enterprises Limited (HCG), one of India’s major cancer care networks, collaborated with Accenture to expedite cancer research and care by leveraging sophisticated AI, such as generative AI and deep learning on multidimensional and multi-omic patient data. This strategic collaboration brings together Accenture’s global expertise and talent in data and AI, including AI/ML, generative AI, and quantum computing, with HCG’s deep clinical insights in oncology to enable early identification and treatment of many types of cancer.
Recent Trends:
Emerging trends include AI integration, immunotherapy innovations, advanced targeted therapies, precision oncology, radiation and surgical techniques, and bispecific antibodies.
The number of clinical trials looking at the potential of modified Natural Killer (NK) cells for cancer treatment has gradually increased over time. There are now more than 20 clinical trials using CAR-NK cells for hematological and solid purposes. Engineered iNK cells provide an easily scaled, off-the-shelf cell treatment. Modifying iNK cells with cytokine signaling and immune-evasion modules will increase their proliferation and durability, boosting the therapeutic efficacy of these therapies. Rapid developments in single-cell sequencing and CRISPR screening promise to expand our understanding of NK cell signaling networks, paving the way for future improvements in NK cell therapeutics that capitalize on NK cells’ beneficial biology.
On September 2024, Vironexis Biotherapeutics, a company dedicated to transforming cancer treatment by pioneering AAV-delivered T-cell immunotherapy, launched its TransJoin Adeno-associated viruses (AAV) gene therapy platform and a pipeline of more than ten product candidates for blood-based cancers, solid tumor metastasis prevention, and cancer vaccine.
Restraints & Challenges:
The issues of scalability and affordability of immunotherapies are numerous and crucial to the advancement of cancer therapy techniques. One of the key concerns is the inherent complexity of the production procedures for these medicines. Cancer immunotherapy can be excessively expensive, limiting access for many patients. The necessity for personalized techniques further impedes immunotherapy scaling. For example, creating patient-derived organoids for drug testing and therapy development has logistical problems.
In addition to logistical and financial constraints, the regulatory landscape complicates the scaling of immunotherapies. The regulatory processes for new medicines, particularly those incorporating genetic alterations like mRNA vaccines and CAR T-cell therapies, are difficult and time-consuming.
Therapy Type Segment Insights and Analysis:
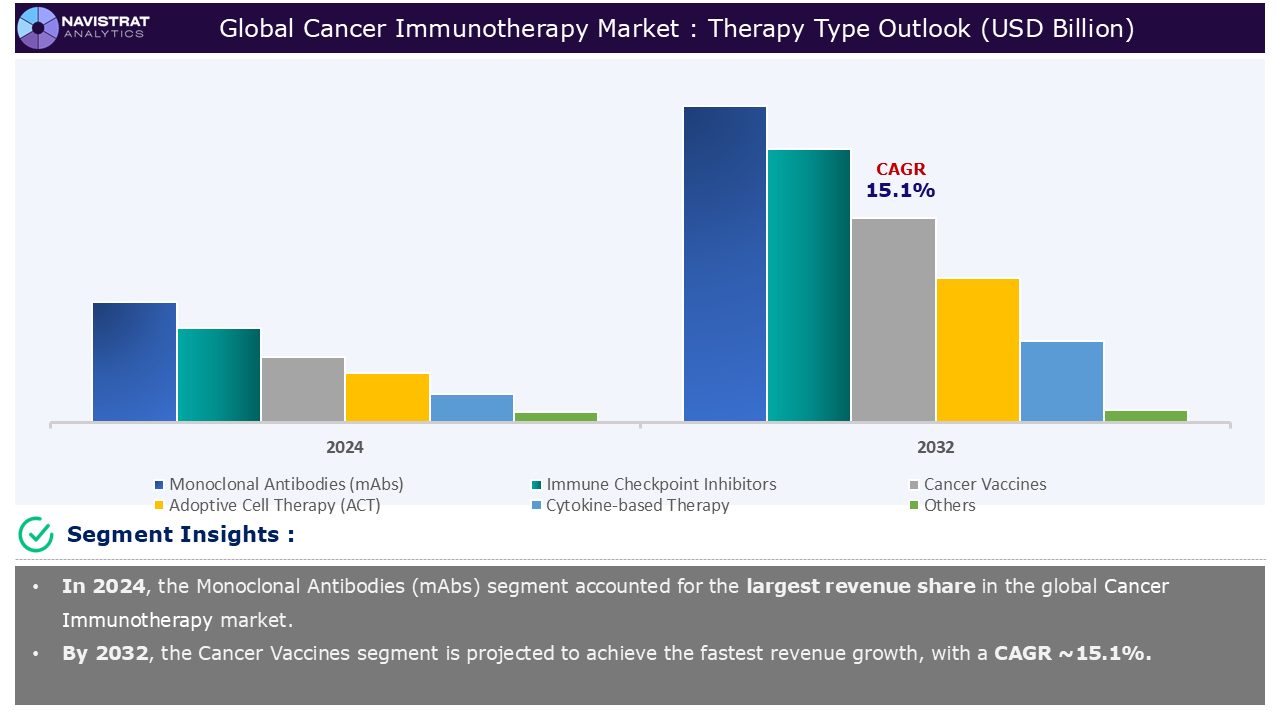
Based on the therapy type, the cancer immunotherapy market is segmented into Monoclonal Antibodies (mAbs), immune checkpoint inhibitors, cancer vaccines, Adoptive Cell Therapy (ACT), cytokine-based therapy, and others.
Monoclonal Antibodies (mAbs) segment contributed the largest market share in 2024. In recent years, mAb immunotherapy has been successfully used to treat and manage a variety of malignancies. Primary solid tumors have been efficiently treated and managed using antibody-chemotherapy conjugates. Researchers have now created monoclonal antibodies (mAbs) that target antigens in cancer cells and block signals that promote tumor growth and invasion. Because they are targeted medicines, their key advantage is that they have fewer side effects than traditional therapies. The significance of mAbs as a therapeutic stem from their great specificity for tumor antigens and many methods of action on these cells to eliminate them.
Combination therapies, integrating monoclonal antibodies with checkpoint inhibitors, CAR T-cell therapy, or small-molecule inhibitors, are showing promising results in improving efficacy and reducing resistance. Advances in personalised medicine enable better patient selection and tailored treatments, maximising therapeutic benefits. Additionally, multispecific antibodies, targeting multiple pathways, are emerging as an effective approach, particularly in cancers with complex immune evasion mechanisms.
On March 2023, BioNTech SE and OncoC4, Inc., a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel biologicals for cancer treatment, announced that they have signed an exclusive worldwide license and collaboration agreement to develop and commercialize ONC-392, OncoC4’s next-generation anti-CTLA-4 monoclonal antibody candidate, as monotherapy or combination therapy in a variety of cancer indications.
Biomarker Type Segment Insights and Analysis:
Based on the biomarker type, the cancer immunotherapy market is segmented into PD-L1 Expression, Tumor Mutational Burden (TMB), Microsatellite Instability (MSI), neoantigen load, and others.
PD-L1 Expression segment contributed the largest market share in 2024. Many researchers have been looking into the PD-1/programmed death-ligand 1 (PD-L1) pathway in recent years because of its outstanding clinical success, long-term response, and low toxicity level. Anti-PD-1/PD-L1 inhibitors have proven to be effective immune checkpoint inhibitors (ICIs) and are quickly becoming the standard treatment for a variety of cancer types. Tumor immunotherapy tries to disrupt the activity of inhibitory immunological checkpoint proteins and enhance T cell activation to induce antitumor immune effects.
On August 2024, Astellas Pharma Inc. announced that the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has approved PADCEV (enfortumab vedotin) for the treatment of adult patients with locally advanced or metastatic urothelial cancer (la/mUC) who have previously received platinum-containing chemotherapy and programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitors.
Indication Segment Insights and Analysis:
Based on the indication, the cancer immunotherapy market is segmented into in breast cancer, lung cancer, colorectal cancer, prostate cancer, ovarian cancer, hematologic malignancies, and others.
Lung cancer segment contributed the largest market share in 2024. According to the American Cancer Society, lung cancer (both small cell and non-small cell) is the second most frequent cancer among men and women in the United States. There will be around 226,650 new instances of lung cancer in the United States in 2025 (110,680 in men and 115,970 in women), as well as approximately 124,730 deaths from lung cancer (64,190 in men and 60,540 in women). Older persons are more likely to develop lung cancer. Most patients diagnosed with lung cancer are 65 or older; only a small minority are under the age of 45. The average age of those diagnosed is around 70.
On August 2024, Johnson & Johnson announced that the FDA has approved RYBREVANT (amivantamab-vmjw) plus LAZCLUZE (lazertinib) for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, as detected by an FDA-approved test. RYBREVANT is an EGFR- and MET-directed bispecific antibody that activates the immune system, while LAZCLUZE is a highly selective, brain-penetrant third-generation oral EGFR TKI.
Geographical Outlook:
Cancer immunotherapy market is strategically segmented by geography to provide a comprehensive understanding of regional market dynamic. Discover demand analysis, emerging trends, and growth opportunities shaping market performance across different region and countries.
North America Cancer Immunotherapy Market:
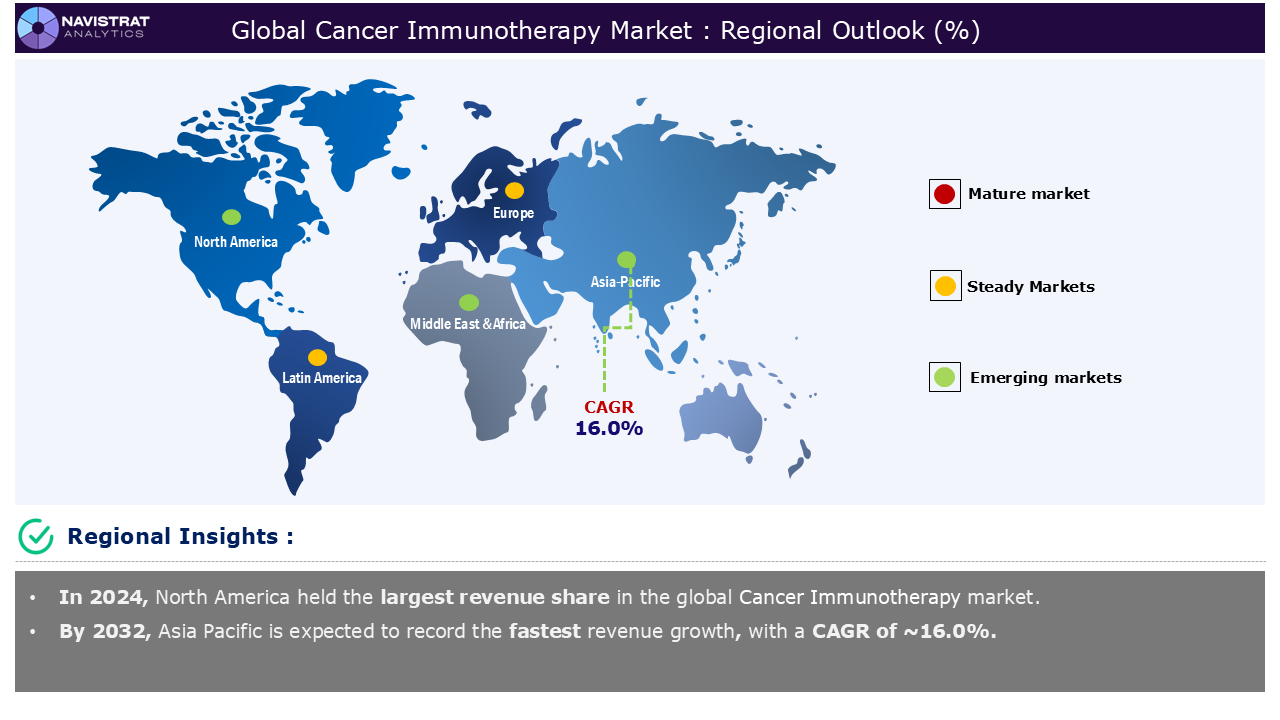
North America is registered to have highest market share in cancer immunotherapy market in 2024. This is mainly due to rising prevalence of cancers worldwide and technological advancements in cell & gene therapies. According to the American Cancer Society, breast cancer is the most frequent cancer in women in the United States, except for skin cancers. It accounts for around 30% (or one in every three) of all new female malignancies each year. The American Cancer Society also predicts that in 2025, around 316,950 new instances of invasive breast cancer in women will be diagnosed, as well as approximately 59,080 new cases of ductal carcinoma in situ (DCIS), about 42,170 women will die from breast cancer.
On February 2025, Eli Lilly and Company and Scorpion Therapeutics, Inc., a private biotechnology firm researching small molecule precision oncology medicines, signed a formal deal for Lilly to purchase Scorpion’s PI3Kα inhibitor programme STX-478. STX-478, a once-daily oral, mutant-selective PI3Kα inhibitor, is being examined in a Phase 1/2 clinical trial for breast cancer and other advanced solid tumours. STX-478 could be the next generation of PI3Kα targeting drugs, selectively targeting the pathway in malignant cells but not healthy cells. This overcomes a critical drawback of current PI3Kα inhibitors.
Asia Pacific Cancer Immunotherapy Market:
Asia Pacific is registered the fastest growth rate during the forecasted period. The region is boosted by rising healthcare infrastructure, rising prevalence of cancers worldwide, and development of next-generation immunotherapies. Advances in next-generation immunotherapies, such as CAR-T cell therapy and ICIs, represent a paradigm change in cancer care. These breakthrough medicines harness the immune system’s ability to recognize and destroy cancer cells, providing new hope, particularly for patients with refractory or advanced disease. The Asia-Pacific area has cemented its place as a premier hub for vaccine research and development, utilizing its cost-effectiveness, diverse demographics, and speedy participant recruitment. With a major proportion of both preventive and therapeutic vaccination studies, the area plays a crucial role in influencing the future of global vaccine innovation.
On November 2024, HUTCHMED has announced that it will receive a milestone payment from Takeda following the pricing clearance and launch of FRUZAQLA (fruquintinib) 1mg/5mg capsules in Japan for patients with previously treated metastatic colorectal cancer. The Japanese Ministry of Health, Labour, and Welfare has approved the manufacture and marketing of FRUZAQLA. FRUZAQLA represents Japan’s first innovative oral targeted medication approved in over a decade to treat metastatic colorectal cancer, regardless of biomarker status. Physicians use FRUZAQLA to treat advanced or recurring colorectal cancer that cannot be cured or surgically removed and has progressed after chemotherapy.
Europe Cancer Immunotherapy Market:
Europe is to register a considerable market share during the forecasted period. This is mainly driven by technological advancements in cell & gene therapies and personalized medicine & biomarker-driven therapies. Every year in Europe, 2.7 million individuals are diagnosed with cancer, and 1.3 million die because of the disease. Digital transformation in healthcare can improve precision medicine by balancing high standards of care with affordability. AI-powered digital solutions can boost patient and family involvement in disease management, potentially shifting healthcare from hospital-based to community-based treatment.
On September 2024, Roche stated that the U.S. Food and Drug Administration (FDA) has approved Tecentriq Hybreza (atezolizumab and hyaluronidase-tqjs), the first and only PD-(L)1 inhibitor for subcutaneous (SC) under the skin injection in the United States. Tecentriq Hybreza can be given subcutaneously in around seven minutes, whereas normal Tecentriq (atezolizumab) infusion takes 30-60 minutes. It will be offered for all IV indications of Tecentriq approved for adults in the United States, including specific types of lungs, liver, skin, and soft tissue cancer.
Competition Analysis:
The cancer immunotherapy market is characterized by a fragmented structure, with several players competing across various segments and regions. List of major players included in the cancer immunotherapy market report are:
- Bristol-Myers Squibb
- Hoffman-La Roche Ltd.
- Novartis AG
- Merck KGaA
- Pfizer Inc
- Eli Lily and Company
- GlaxoSmithKline plc
- Johnson & Johnson Services, Inc.
- Amgen Inc.
- Incyte Corporation
- Moderna, Inc.
- Bayer AG
- Xilio Therapeutics
- Takeda Pharmaceutical Company Limited
- Gilead Sciences, Inc.
- Sanofi
Strategic Developments in Cancer Immunotherapy Market:
- In June 2025, BioNTech SE and Bristol Myers Squibb announced a global co-development and co-commercialization agreement for BioNTech’s investigational bispecific antibody BNT327 across a wide range of solid tumor types. Under the agreement, BioNTech and BMS will collaborate to widen and expedite the development of this clinical candidate. According to the terms of the agreement, the firms will collaborate to develop and market BNT327, including both as a monotherapy and in combination with other treatments. Both businesses have the option to independently develop BNT327 in additional indications and combinations, including combinations with proprietary pipeline assets.
- In March 2024, Mirvetuximab soravtansine-gynx (Elahere, ImmunoGen, Inc.) has been approved by the FDA for adult patients with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have had one to three prior systemic treatments. Patients are selected using an FDA-approved test. Mirvetuximab soravtansine-gynx was previously granted expedited approval for this indication.
- In November 2023, RefleXion Medical, a therapeutic cancer firm, has announced the first close of a USD 105 million equity raise spearheaded by The Rise Fund, TPG’s multi-sector global impact investing strategy. These fresh financing will enable RefleXion to expand the commercialization of its groundbreaking SCINTIX medication for treating all stages of specified solid tumor malignancies, including metastatic disease. SCINTIX is the first and only technology that converts an injected radiotracer into real-time signals that control autonomous radiotherapy.
Key Advantages for Stakeholders:
Navistrat Analytics’ industry report provides an in-depth quantitative analysis of various market segments, historical and current trends, market forecasts, and dynamics within the global market. The historical years covered in this report are 2022 to 2023, with 2024 serving as the base year for market size calculations. The forecast period extends from 2025 to 2032.
The report includes an executive summary and a comprehensive overview of market drivers, restraints, opportunities, and challenges (DROC), along with insights into regulatory standards. It features detailed analyses such as PORTER’s Five Forces, SWOT, and PESTLE, as well as assessments of technological trends and the competitive landscape.
PORTER’s Five Forces analysis helps stakeholders evaluate the impact of new entrants, competitive rivalry, supplier power, buyer power, and substitution threats, enabling them to assess the level of competition and the attractiveness of the global market. The competitive landscape provides stakeholders with a clear understanding of the current market positions of key players, offering valuable insights into their competitive environment.
Scope And Key Highlights of the Cancer Immunotherapy Market Report:
| Report Features | Details |
| Market Size in 2024 | USD 220.94 Billion |
| Market Growth Rate in CAGR (2025–2032) | 13.6% |
| Market Revenue forecast to 2032 | USD 615.64 Billion |
| Base year | 2024 |
| Historical year | 2022-2023 |
| Forecast period | 2025-2032 |
| Report Pages | 450 |
| Segments covered |
|
| Regional scope |
|
| Country Scope |
|
| Key Market Players |
|
| Delivery Format | Reports are delivered in PDF format via email. |
| Customization scope | Request for Customization |
The Cancer Immunotherapy market report offers a detailed analysis of market size, including historical revenue (in USD Billion) data for 2022-2023 and revenue forecasts for 2025-2032 across the following segments:
- Therapy Type Outlook (Revenue, USD Billion; 2022-2032)
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Biomarker Type Outlook (Revenue, USD Billion; 2022-2032)
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Indication Outlook (Revenue, USD Billion; 2022-2032)
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- End-Use Outlook (Revenue, USD Billion; 2022-2032)
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Regional Outlook (Revenue, USD Billion; 2022-2032)
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- U.K.
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of MEA
- North America
Frequently Asked Questions (FAQ) about the Cancer Immunotherapy market report
The market size of cancer immunotherapy market was 220.94 billion in 2024.
The market size of cancer immunotherapy market is expected to register compound annual growth rate (CAGR) of 13.6% over the forecast period.
Rising prevalence of cancers worldwide, technological advancements in cell & gene therapies, and personalized medicine & biomarker-driven therapies are major key factors driving the market revenue growth of the cancer immunotherapy market.
High production and treatment costs and complex manufacturing and supply chain requirements are key limiting factors driving the market.
Asia Pacific account for fastest revenue growth of 16.0%.
Monoclonal Antibodies (mAbs) is the major leading segment of cancer immunotherapy market in terms of therapy type.
- Market Definition
- Research Objective
- Research Methodology
- Research Design
- Data Collection Indications
- Primary
- Secondary
- Market Size Estimation
- Top-down Indication
- Bottom-up Indication
- Forecasting Methodology
- Tools and Models Used
- Market Overview and Trends
- Market Size and Forecast
- Industry Analysis
- Market Driver, Restraints, Opportunity, and Challenges (DROC) Analysis
- Market Drivers
- Rising prevalence of cancers worldwide
- Technological advancements in cell & gene therapies
- Personalized medicine & biomarker-driven therapies
- Market Restraints
- High production and treatment costs
- Complex manufacturing and supply chain requirements
- Market Opportunities
- Development of next-generation immunotherapies
- Integration of ai & big data in drug discoveries
- Shift Toward Allogeneic Cell Therapies
- Market Challenges
- Biological complexity & heterogeneity of cancer
- Commercialization Barriers and Intellectual Property (IP) Conflicts
- Regulatory Landscape
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
- Strategic Insights
- Porter’s Five Forces Analysis
- PESTLE Analysis
- Price Trend Analysis
- Value Chain Analysis
- Technological Trends
- Recent Developments
- Funding
- Merger and Acquisition
- Expansion
- Partnership and Collaboration
- Product/ Service Launch
- Therapy Type Market Revenue Estimates and Forecasts, 2022-2032
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Biomarker Type Market Revenue Estimates and Forecasts, 2022-2032
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Indication Market Revenue Estimates and Forecasts, 2022-2032
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- End-Use Market Revenue Estimates and Forecasts, 2022-2032
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Cancer Immunotherapy Market Revenue Estimates and Forecasts by Region, 2022-2032, USD Billion
-
- North America
- North America Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- North America Cancer Immunotherapy Market By Biomarker Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- North America Cancer Immunotherapy Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- North America Cancer Immunotherapy Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- North America Cancer Immunotherapy Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Billion
- United States
- Canada
- Mexico
- North America Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- North America
- Europe
- Europe Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Europe Cancer Immunotherapy Market By Biomarker Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Europe Cancer Immunotherapy Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- Europe Cancer Immunotherapy Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Europe Cancer Immunotherapy Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Billion
- Germany
- United Kingdom
- France
- Italy
- Spain
- Benelux
- Nordic Countries
- Rest of Europe
- Europe Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Asia-Pacific
- Asia-Pacific Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Asia-Pacific Cancer Immunotherapy Market By Biomarker Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Asia-Pacific Cancer Immunotherapy Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- Asia-Pacific Cancer Immunotherapy Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Asia-Pacific Cancer Immunotherapy Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Billion
- China
- India
- Japan
- South Korea
- Oceania
- ASEAN Countries
- Rest of Asia-Pacific
- Asia-Pacific Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Latin America
- Latin America Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Latin America Cancer Immunotherapy Market By Biomarker Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Latin America Cancer Immunotherapy Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- Latin America Cancer Immunotherapy Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Latin America Cancer Immunotherapy Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Billion
- Brazil
- Rest of Latin America
- Latin America Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Middle East & Africa
-
- Middle East & Africa Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Monoclonal Antibodies (mAbs)
- Immune Checkpoint Inhibitors
- Cancer Vaccines
- Adoptive Cell Therapy (ACT)
- Cytokine-based Therapy
- Others
- Middle East & Africa Cancer Immunotherapy Market By Biomarker Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- PD-L1 Expression
- Tumor Mutational Burden (TMB)
- Microsatellite Instability (MSI)
- Neoantigen Load
- Others
- Middle East & Africa Cancer Immunotherapy Market By Indication, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer
- Hematologic Malignancies
- Others
- Middle East & Africa Cancer Immunotherapy Market By End-Use, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Hospitals
- Cancer Research Institutes
- Specialty Clinics
- Academic & Research Centers
- Biopharmaceutical Companies
- Middle East & Africa Cancer Immunotherapy Market Revenue Estimates and Forecasts by Country, 2022-2032, USD Billion
- GCC Countries
- South Africa
- Israel
- Turkey
- Rest of Middle East & Africa
- Middle East & Africa Cancer Immunotherapy Market By Therapy Type, Market Revenue Estimates and Forecasts, 2022-2032, USD Billion
- Market Share Analysis
- Revenue Market Share by Key Players (2023-2024)
- Analysis of Top Players by Market Presence
- Competitive Matrix
- Competitive Strategies
- Mergers and Acquisitions
- Partnerships and Collaboration
- Investment and Fundings
- Agreement
- Expansion
- New Product/ Services Launches
- Technological Innovations
- Bristol-Myers Squibb
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Hoffman-La Roche Ltd.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Novartis AG
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Merck KGaA
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Pfizer Inc
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- GlaxoSmithKline plc
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Eli Lily and Company
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Johnson & Johnson Services, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Amgen Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Incyte Corporation
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Moderna, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Bayer AG
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Xilio Therapeutics
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Takeda Pharmaceutical Company Limited
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Gilead Sciences, Inc.
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis
- Sanofi
- Company Overview
- Financial Insights
- Product/ Services Offerings
- Strategic Developments
- SWOT Analysis

